Why Do We Feel Heat From The Sun?
The Sun is the primary source of heat and light for the Earth. Every day we feel the warmth of the Sun on our skin, but how exactly does the heat get from the Sun to us?
This article will provide an overview of the process, covering the high temperature at the surface of the Sun, how it emits radiation across space, how that radiation reaches and interacts with the Earth’s atmosphere and surface, and how the heat is ultimately transferred to objects on Earth like our skin that allows us to perceive the warmth.
The Sun’s Temperature
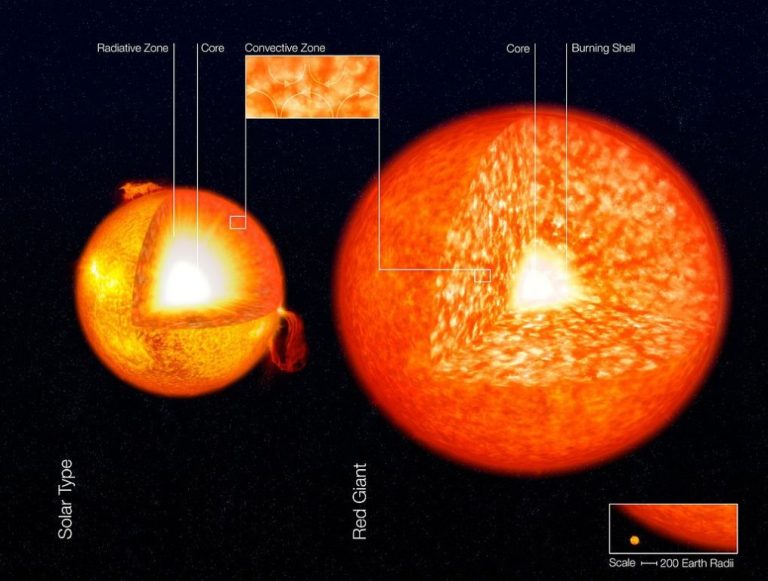
The surface of the sun has an extremely high temperature, averaging around 10,000°F. However, the core of the sun is even hotter, with temperatures exceeding 27 million °F. This extreme heat is a result of nuclear fusion reactions occurring at the sun’s core.
The sun produces energy through a process called nuclear fusion. Atoms of hydrogen and helium gas in the sun’s core are pushed together at very high temperatures and pressures, causing them to fuse into helium. This fusion reaction releases an enormous amount of energy in the form of gamma rays. As the gamma rays travel outward from the core, they continuously collide with surrounding atoms and gradually lose energy, converting into lower energy photons in the visible light spectrum.
By the time the photons reach the sun’s surface and radiate out into space, the temperature has cooled to about 10,000°F. However, the core remains extremely hot with temperatures high enough to sustain continuous fusion reactions. This thermonuclear fusion at the core is the source of the sun’s staggering luminosity and heat that impacts us here on Earth.
Radiation from the Sun
The sun is an extremely hot ball of gases and plasma. At its core, the temperature reaches 15 million degrees Celsius. This high temperature causes the gases in the sun’s core to undergo nuclear fusion, which releases enormous amounts of energy. Some of this energy is emitted from the sun’s surface in the form of electromagnetic radiation.
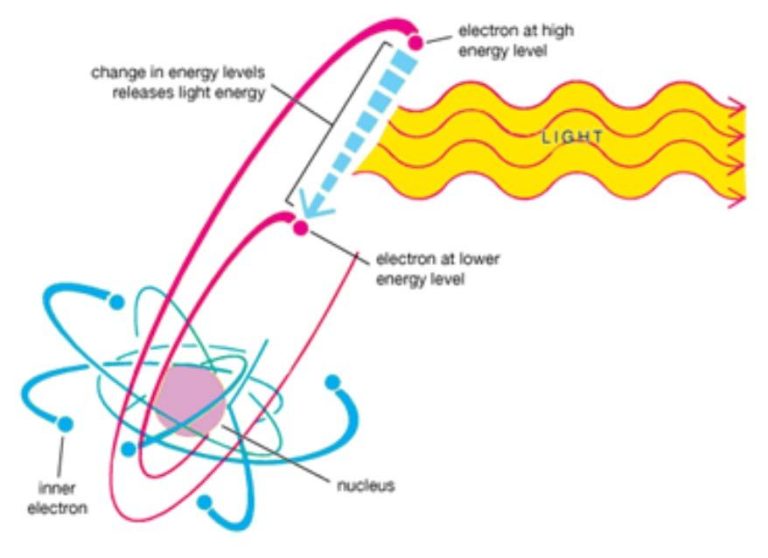
Radiation is emitted at all frequencies, or wavelengths, across the electromagnetic spectrum. This includes radio waves, microwaves, infrared, visible light, ultraviolet, x-rays, and gamma rays. However, most of the sun’s radiation is concentrated in the visible and ultraviolet portion of the spectrum. The peak emission occurs at about 500 nanometers, which we perceive as greenish-blue light.
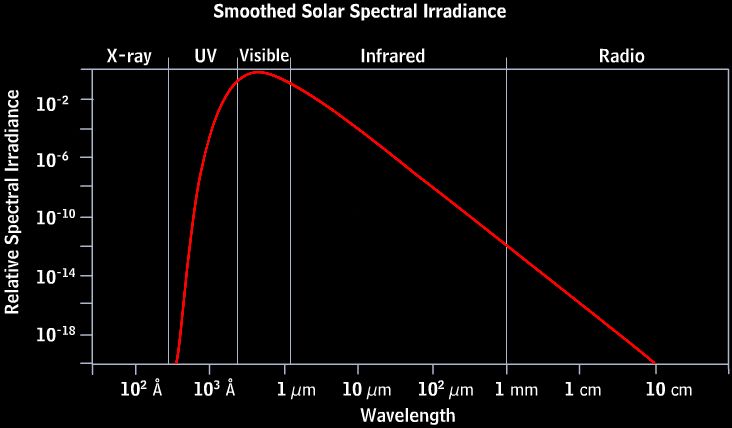
This wide spectrum of radiation streams outward in all directions from the sun’s surface into space. Some of it eventually reaches Earth, providing the light and heat that makes life possible on our planet. The sun’s high temperature is therefore the ultimate source of nearly all radiation emitted from our closest star.
Solar Radiation Reaching Earth
The Sun emits a broad spectrum of radiation, ranging from high-energy x-rays and ultraviolet rays to lower energy visible light and infrared radiation. As this radiation travels the 150 million kilometers from the Sun to Earth, different components are filtered or absorbed by gases in the upper atmosphere.
Ultraviolet rays, x-rays, and most gamma rays coming from the Sun do not penetrate Earth’s atmosphere. Ozone gas strongly absorbs solar ultraviolet radiation, protecting living organisms on Earth’s surface from these highly energetic and damaging wavelengths.
Visible sunlight, along with infrared and some ultraviolet rays, do make it through the atmosphere and reach Earth’s surface. Of the solar energy that reaches the top of the atmosphere, about half reaches the ground as visible light, plus infrared and ultraviolet radiation. Clouds reflect some of this incoming solar radiation back to space.
The exact spectrum of solar energy reaching a given spot on Earth depends on factors like time of day, season, cloud cover, smog levels, and location. But in general, the solar radiation that does reach the surface provides a continual source of energy that powers life processes and drives Earth’s climate system.
Absorption of Radiation
When solar radiation reaches Earth, not all surfaces absorb the radiation at the same rate. The absorption of radiation depends on the material’s albedo, which is a measure of how much radiation it reflects. Surfaces with a low albedo absorb more radiation, while surfaces with a high albedo reflect more.
For example, snow and ice have a high albedo – they reflect up to 90% of radiation. This means they absorb little solar radiation and remain cool. In contrast, forests and oceans have a low albedo, absorbing up to 95% of radiation and heating up more. Pavements and buildings in urban areas also tend to have a low albedo due to their dark colors, absorbing up to 90% of radiation.
The differential absorption of solar radiation by varying surfaces helps regulate Earth’s climate. If the planet was covered in ice and snow, little radiation would be absorbed to warm the atmosphere. The mix of high and low albedo surfaces provides the necessary energy absorption while also allowing reflection to avoid overheating.
Conversion to Thermal Energy
When solar radiation is absorbed by an object, the energy from the radiation is converted into thermal energy or heat. This occurs through a process called absorption. As photons of light strike the object, they are either reflected, transmitted, or absorbed. The photons that are absorbed transfer their energy to the atoms and molecules that make up the object.
This influx of energy causes the atoms and molecules to vibrate and move faster. As they speed up, the thermal energy or temperature of the object increases. The higher vibrational motions of the atoms and molecules generate heat. This conversion of radiant solar energy into thermal energy is known as the photothermal effect. The amount of heat produced depends on the material, color, and nature of the surface. Darker and more opaque materials tend to absorb more radiation and convert it into heat.
In this way, the radiant energy from the Sun is transformed into thermal energy within objects it shines upon. This added heat is what we perceive as warmth coming from the Sun. The photothermal effect allows sunlight to be a source of thermal energy that warms the Earth.
Heat Transfer to Objects
When sunlight reaches the Earth’s surface, the ground and objects on the ground absorb some of the radiation in the form of heat. Heat then transfers from these heated surfaces to adjacent objects through the process of conduction. Conduction is the transfer of heat between objects that are in direct contact with each other. The better the conducting material, the faster heat will transfer through it. Metals are very good conductors while materials like wood and plastic are poor conductors.
As an example, when sunlight shines on a metal park bench, the metal absorbs heat from the radiation. A person sitting on the bench will feel the bench grow warm because heat is conducted from the metal into their body. The metal atoms vibrate faster as they gain energy from the radiation, and these vibrating atoms collide with atoms in the surrounding air or anything touching the bench, transferring thermal energy. The bench also conducts heat downward into the concrete below it, heating the ground. Conduction allows absorbed radiant heat from the Sun to transfer through and between objects, warming them up.
Heat Transfer to Air
The warm surfaces of the Earth also heat the surrounding air through the process of convection. As surfaces absorb solar radiation and become warmer, the air molecules directly adjacent to the surface gain kinetic energy and start to move faster and spread apart. This makes the heated air less dense than the cooler air above it. The warm air then rises since it is now less dense than the surrounding cooler air. As the warm air rises, cooler air moves in to take its place, gets heated, and continues the convection cycle.
The constant cycling of warm air rising and cooler air sinking causes the transfer of heat from Earth’s surfaces to the atmosphere. This transfer of thermal energy by the movement of fluids like air and water is known as convection. It’s why we feel a warm breeze on a hot summer day – the ground has heated the surrounding air which then rises, flows, and transfers that warmth to us.
Perception of Heat
The heat that is transferred to the air and surroundings ultimately leads to our perception of feeling warmer. There are three main heat transfer mechanisms at play that cause this warming effect on people:
Conduction occurs when direct contact between your skin and a warm object, like a sun-heated bench or rock, causes heat to be conducted to your body. The greater the temperature difference between your skin and the object, the faster heat is transferred.
Convection happens when the heated air around you rises and is replaced by cooler air below. This circulation pattern brings the warm air into contact with your skin, transferring heat by convection.
Radiation is when infrared radiation emitted by hot surfaces like sand, pavement, or walls is absorbed by your skin directly. This infrared radiation carries heat energy that is converted into thermal energy when absorbed.
Through these three heat transfer mechanisms—conduction, convection, and radiation—the thermal energy from solar radiation heats the air and surroundings, ultimately leading to our perception and sensation of feeling warmer on a sunny day.
Conclusion
In conclusion, we feel heat from the Sun due to a multi-step process:
The Sun produces immense heat and light energy through nuclear fusion reactions at its extremely high core temperature of 15 million degrees Celsius.
This energy radiates outward from the Sun in the form of electromagnetic radiation across a spectrum of wavelengths, including ultraviolet light, visible light, and infrared radiation.
Some of this solar radiation reaches the Earth after traveling around 150 million kilometers through space. The Earth’s atmosphere allows visible light and ultraviolet radiation to pass through, while absorbing some of the infrared radiation.
When the incoming solar radiation encounters objects on Earth, the radiation is absorbed and converted into thermal energy, increasing the temperature of the objects it interacts with.
This heat is then transferred to the surrounding air molecules through conduction and convection, warming the air around hotter objects.
The warming of objects and air makes us feel hotter when we are exposed to this heated environment. Our bodies sense heat through thermoreceptors in our skin that detect infrared radiation and increases in air temperature.
In summary, the original heat source is the Sun’s core, which radiates energy to Earth that is then absorbed and converted into thermal energy, heating objects and air which we perceive as warmth. This explains the multi-step process of how we feel the heat from the Sun.