Where Does Electric Charge Come From?
Electric charge is a fundamental property of matter that exists in two types – positive and negative. Although invisible, electric charge plays a critical role in almost every aspect of our daily lives. It allows atoms and molecules to connect to form the tangible objects and materials around us. It causes static electricity, lightning strikes, and electrical currents. Understanding electric charge is key to harnessing the power of electricity that illuminates our homes, computes our data, and drives communications.
In this article, we will explore what exactly electric charge is, the different ways it arises, and some of its common manifestations in our world.
Atomic Structure
The protons in an atom’s nucleus carry a positive electric charge, while the electrons that orbit the nucleus carry a negative electric charge. Atoms are made up of three main particles – protons, neutrons, and electrons. Protons and neutrons cluster together to form the nucleus at the center of the atom. Protons have a positive electric charge (+), electrons have a negative electric charge (-), and neutrons have no electric charge (neutral).
The number of protons in an atom determines its atomic number and identifies the specific element. For example, hydrogen atoms have 1 proton, helium atoms have 2 protons, carbon atoms have 6 protons, and so on. The number of electrons orbiting the nucleus is equal to the number of protons, resulting in a neutral charge. However, electrons can be gained or lost to form charged ions. The gain or loss of electrons creates a positive or negative electric charge.
Ionization
Atoms can gain or lose electrons through a process called ionization. This causes them to become positively or negatively charged ions. Ionization can occur through several methods:
– Thermal ionization: Heating an atom can provide enough energy for an electron to break free from the atom’s outer shell and leave behind a positively charged ion.
– Photoionization: Radiation like ultraviolet light can strike an atom and eject an electron, creating a positive ion.
– Collisional ionization: Collisions between atoms or molecules can knock electrons loose, forming ions. This often happens in flames or plasma.
– Electrical discharges: Strong electrical fields can pull electrons out of atoms, ionizing the atoms. Lightning is an example of electrical discharge ionization.
– Chemical ionization: Chemical reactions can transfer electrons between atoms and molecules, leading to ionization.
– Electron capture: Some atoms or molecules can capture stray electrons, becoming negative ions in the process.
The loss or gain of electrons is key to creating electric charge at the atomic level. When an atom loses electrons it becomes positively charged, whereas gaining electrons leads to a negative charge. This ionization process provides the source of electric charges that drive many electrical phenomena.
Triboelectric Effect
The triboelectric effect is one way that objects can gain or lose electrons and thus build up static electric charge. When two different materials come into contact and are then separated, electrons can transfer from one material to the other due to differences in their electron affinity. This causes one material to become positively charged and the other negatively charged.
For example, when a glass rod rubs against a piece of silk, electrons transfer from the silk to the glass rod. The glass rod gains extra electrons and becomes negatively charged, while the silk loses electrons and becomes positively charged. The more rubbing and contact, the greater the charge buildup. This is because the materials have different tendencies to attract or repel electrons.
Materials are often listed in a triboelectric series, which ranks their tendency to become positively or negatively charged. Materials higher on the list have a greater affinity for electrons and tend to become negatively charged when in contact with materials lower on the list. Rubbing glass and silk together produces a greater charge difference than rubbing two materials closer together on the series.
The triboelectric effect is strongest when rubbing two very different materials together, like metal on fabric or plastic on fur. Insulating materials also tend to develop stronger charges. The material properties, surface smoothness, and other factors can influence the effect. Overall, the triboelectric effect demonstrates that everyday contact between objects can lead to charge transfer and buildup.
Electrostatic Induction
Electrostatic induction is the process by which electrical charge is redistributed in an object. This occurs when a neutral object is brought near a charged object. The charged object will exert an electrostatic force that causes the charges in the neutral object to rearrange.
Specifically, the presence of the charged object will cause the charges in the neutral object to polarize. The polarized charges will align so that the opposite charges are closest to the charged object. This results in one side of the object becoming positively charged and the opposite side becoming negatively charged.
For example, if a positively charged object is brought near an initially neutral metal sphere, the negative charges (electrons) in the sphere will be attracted toward the side closest to the positively charged object. The other side of the sphere will develop a net positive charge. Overall the sphere is still neutral, but the charges have rearranged due to the proximity of the external charged object.
This redistribution of charge without transfer of charge between objects is known as electrostatic induction. It demonstrates that the presence of nearby charges can exert forces that polarize and redistribute charges in neutral objects. This process plays an important role in phenomena like static electricity.
Electrical Currents
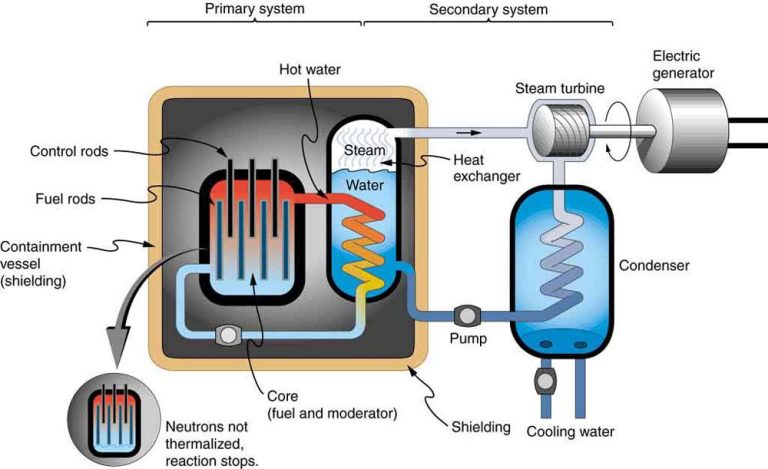
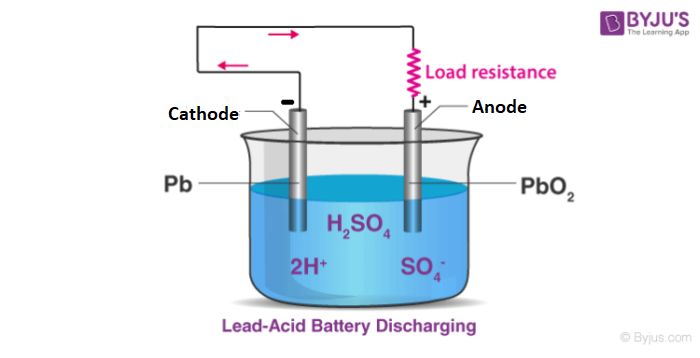
Electric currents are the flow of electric charge through a conductor, such as a wire or circuit. Charge flows when there is a voltage difference between two points in a circuit, creating an electric field within the conductor that exerts force on the charges and causes them to move.
In metals like copper, electric current is carried by electrons. Metals contain many loosely bound, or “free” electrons that can move through the material. When a voltage is applied, the electric field propels the free electrons in one direction through the circuit. This electron flow is the electric current.
Current flows in closed loops through a circuit. It originates from the negative terminal of the voltage source like a battery, flows through the various circuit components powered by the source, then returns to the positive terminal to complete the loop. The current remains continuous as long as the voltage source maintains the electric field to keep driving electron flow.
The strength of the current is measured in amperes (amps) and depends on the voltage applied and the resistance of the circuit. Higher voltage causes more current to flow. Resistance impedes current flow somewhat like friction, and can be increased to reduce current. Conductors like metals have very low resistance and allow more current to flow easily. Insulators like rubber have extremely high resistance and block the flow of current.
In summary, electric currents result from the organized motion of charges through a conductor. Applying a voltage creates an electric field that causes the charges to flow in a continuous loop through a complete circuit. This charge flow constitutes the electric current that powers electrical devices and systems. Understanding current flow allows us to harness electricity for human purposes.
Static Electricity
Static electricity refers to electric charges that build up on surfaces through contact and friction. When two surfaces rub against each other, electrons can transfer from one surface to the other, leaving one surface positively charged and the other negatively charged. This is known as the triboelectric effect. Certain materials like plastic, rubber, and fabric are prone to building up static charges more easily than others.
As surfaces continue to rub together, more and more electrons can transfer, building up larger static charges. These charges remain static on the surface until they find a path to ground or neutralize with opposite charges. This is why you may get a small electric shock when touching a doorknob after walking across carpet. The friction from your feet walking across the carpet stole electrons, leaving your body positively charged. When you touch the doorknob, the excess positive charges flow to the grounded metal, giving you a shock.
Static charges also build up through induction. If a charged object is brought near an uncharged conductor, it will rearrange electrons in the conductor. Electrons get pushed to the side farthest from the charged object, leaving the other side with a net opposite charge. For example, if a positively charged item nears a neutral metal object, electrons in the metal will drift to the far side, inducing a net positive charge on the near side. This is due to the attractive and repulsive forces between opposite and like charges.
In summary, surfaces build up static electricity through contact and induction. Friction from rubbing causes electrons to transfer between surfaces, leaving net positive and negative static charges. Meanwhile, the presence of charged objects can induce static charges without even requiring contact. Controlling static buildup is important for electronics, manufacturing, safety, and other applications.
Lightning
Lightning is one of the most dramatic and visible forms of electric discharge. It occurs when charge builds up within storm clouds, leading to a massive electrical spark between the clouds or from a cloud to the ground.
Inside storm clouds, wind and moisture help create separation of electrical charge. Positively charged ice crystals tend to rise to the top of the cloud, while negatively charged hail and rain droplets fall to the bottom. This creates a charged dipole, with the top of the cloud becoming positively charged and the bottom becoming negatively charged.
As more and more charge is separated, the electrical potential between the top and bottom of the cloud keeps increasing. Once the potential exceeds the dielectric strength of the air, a massive discharge occurs in the form of lightning. This sudden release of electric current briefly equalizes the charge within the cloud via the creation of an ionized channel or leaders from one part of the cloud to another.
Most cloud-to-ground lightning originates from the negatively charged bottom of the cloud, traveling down towards positively charged ground features like mountains and trees. However, some storms exhibit “positive lightning” from the upper positive part of the cloud down towards negatively charged ground areas.
In summary, the separation of charge between rising and falling water and ice particles creates increasing electrical potential within clouds, until dielectric breakdown results in a massive lightning discharge and equalization of charge.
Piezoelectricity
Piezoelectricity refers to the ability of certain materials to generate an electrical charge in response to applied mechanical stress. This phenomenon was discovered in 1880 by French physicists Jacques and Pierre Curie. They found that crystals such as quartz, tourmaline, and Rochelle salt would produce an electrical potential difference when subjected to pressure or bending.
The piezoelectric effect occurs due to the crystalline structure of certain materials. These materials have molecular dipole moments that are oriented in an asymmetric manner. When the material undergoes deformation from an applied stress, the positive and negative charge centers within the crystal are displaced, causing a polarization of charge. This charge separation results in a voltage difference across the material. The amount of charge produced is proportional to the mechanical stress applied.
The piezoelectric effect works in reverse as well. Applying an electric field to a piezoelectric material induces a mechanical deformation proportional to the strength of the field. This property allows piezoelectric materials to be used as sensors and actuators in a wide variety of applications.
Piezoelectric materials generate charge through mechanical stress and vibration. This makes them useful for applications such as spark ignition, force and strain measurement, high voltage generation, and more. The piezoelectric effect is utilized in devices ranging from clocks, grills, and washing machines to advanced sensors and precision positioning equipment.
Conclusion
In summary, electricity and electric charge ultimately originate at the atomic level. Atoms consist of protons (positively charged particles), electrons (negatively charged particles), and neutrons (no charge). Ionization occurs when an atom gains or loses electrons, creating a net electric charge. Chemical reactions, friction, heat, and other processes can ionize atoms and molecules, separating positive and negative charges.
Some of the main ways electric charge is generated include:
- The triboelectric effect – rubbing certain materials together exchanges electrons, leaving two charged surfaces.
- Electrostatic induction – bringing a charged object near an uncharged conductor causes separation of charge.
- Flow of electric currents – moving electrons constitute an electric current, which can charge objects.
- Piezoelectric effect – squeezing or compressing certain crystals generates charge separation.
- Lightning – build up of charge differences in storm clouds and between earth leads to lightning discharge.
Overall, electricity arises naturally through a variety of atomic, molecular, and physical processes. Understanding the origins of electric charge provides key insights into the nature of electricity and its role across science and technology.