Where Do You See Potential Energy?
Potential energy is the energy that an object has because of its position or state. For example, a ball held at a height above the ground has gravitational potential energy due to the Earth’s gravity acting upon it. As the ball falls, this potential energy gets converted into kinetic energy – the energy of motion. Potential energy comes from forces between two or more objects that depend on their positions. Common types of potential energy include gravitational, elastic, chemical, nuclear, and electrostatic potential energy.
Potential energy is not the same as kinetic energy. Kinetic energy is the energy of motion that an object has due to its movement. Potential energy is stored energy due to an object’s position in a force field. While kinetic energy is directly related to motion, potential energy is only released when the object starts moving. This allows potential energy to be stored for later use.
Understanding potential energy is important, as there are many examples of it around us. Identifying and harnessing sources of potential energy allows us to perform useful work. For example, dams use the gravitational potential energy of water held at a height to generate electricity through turbines. The study of potential energy also explains behaviors we observe in nature, like why objects fall or bounce.
Gravitational Potential Energy
Gravitational potential energy is the energy stored in an object due to its position or height relative to the ground or a lower position. This energy comes from the gravitational attraction between the object and Earth. The higher the object is above the ground, the greater its gravitational potential energy.
Some examples of gravitational potential energy in everyday life include:
- A book sitting on a high shelf has more gravitational potential energy than the same book sitting on a coffee table.
- A rollercoaster at the top of a hill contains a large amount of gravitational potential energy which gets converted to kinetic energy as it moves downhill and gains speed.
- Water held behind a dam has gravitational potential energy that can be converted into electricity as it flows downhill through turbines.
- A rock balancing at the edge of a cliff contains gravitational potential energy relative to the ground below.
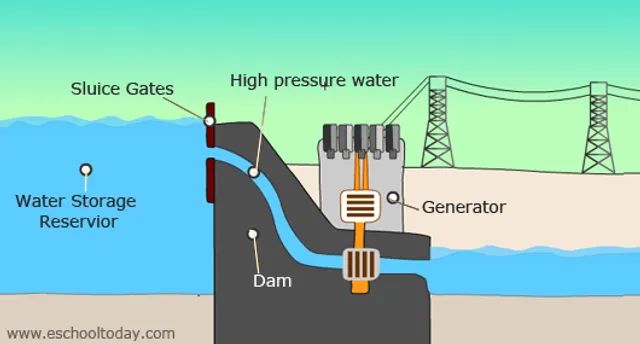
In each case, the object high up has the capacity to do work as it falls down. The gravitational potential energy gets converted to kinetic energy as the object accelerates under gravity. This energy can then be harnessed to perform useful work.
Elastic Potential Energy
Elastic potential energy exists within objects that can be stretched or deformed. When a stretched elastic object is released, the restoring force will cause it to return to its original shape, converting the elastic potential energy into kinetic energy. Some common examples of elastic potential energy include:
- Stretched springs – The coils of a spring get displaced when it is stretched or compressed, storing elastic potential energy. When released, the spring converts this energy into kinetic energy as it returns to its original shape.
- Stretched rubber bands – Rubber can be stretched and deformed, building up elastic potential energy. Releasing the rubber band converts this energy into the kinetic energy of the band contracting.
- Bent bows – Drawing back on a bow deforms the limbs, storing elastic potential energy. This energy is transferred to the arrow upon release, giving it kinetic energy.
- Compressed gases – Compressing gases into a smaller volume increases the elastic potential energy. When released, the gas will rapidly expand, releasing the stored energy.
In all cases, the elastic potential energy results from the temporary deformation of the object’s shape. The restoring force of the deformed material drives its return back to equilibrium, converting the potential energy into kinetic energy.
Chemical Potential Energy
Chemical potential energy is energy stored in the bonds between atoms and molecules. This energy can be released in chemical reactions when the bonds are broken or rearranged. Some common examples of chemical potential energy include:
- Batteries – The chemical reactions that take place in batteries release electrons, generating an electric current. The chemicals in the battery have potential energy that gets converted to electricity.
- Food – Food contains chemical energy in the bonds of molecules like carbohydrates, fats and proteins. This potential energy is released when we digest food and break down those molecules.
- Fuel – Like food, fuels like gasoline, natural gas and propane contain chemical potential energy in their molecular bonds. This energy is released in combustion reactions when the fuels are burned.
Chemical potential energy depends on the types of molecules and bonds involved. Stronger molecular bonds (like triple bonds between carbon atoms) require more energy to break and therefore contain more potential energy. The more potential energy stored in a chemical system, the more useful energy can be released in chemical reactions.
Nuclear Potential Energy
Nuclear potential energy is the potential energy stored in the nucleus of an atom. It is the energy that holds the nucleus together. There are two main types of nuclear potential energy:
- Nuclear fission – This involves splitting the nucleus of a large atom into smaller nuclei. Fission releases energy because the binding energy per nucleon is greater for smaller nuclei than larger nuclei. For example, uranium-235 and plutonium-239 can undergo nuclear fission to produce energy.
- Nuclear fusion – This involves combining two small nuclei together to form a larger nucleus. Fusion releases energy because the binding energy per nucleon is greater for larger nuclei. For example, nuclear fusion powers the sun by fusing hydrogen nuclei into helium.
Nuclear potential energy is millions of times greater than chemical potential energy. That’s why nuclear power plants are able to produce so much energy from very small amounts of nuclear fuel. However, nuclear fission and fusion reactions are difficult to control.
Electrostatic Potential Energy
Electrostatic potential energy is the potential energy from the build up of electric charge between two objects. It arises from the electrostatic force exerted by the electric field. When two oppositely charged objects are brought close together, work must be done against the electrostatic force to move them, giving them a potential energy.
A common example of electrostatic potential energy can be seen in charged capacitors. Capacitors are devices that store electric charge. As charge builds up on the capacitor’s two conductors, it creates an electric field and voltage difference between them. Moving additional charge onto the conductors requires work against the electrostatic forces, which is stored as potential energy in the increasing electric field. The amount of electrostatic potential energy stored in a capacitor is proportional to the square of the voltage across it. This energy can be released when the capacitor discharges through a circuit, converting the potential energy into electricity.
Other examples of electrostatic potential energy include the buildup of static electricity on clothes, lightning discharges between clouds and the ground, and particle accelerators like Van de Graaff generators. Manipulating electrostatic potential energy allows us to store charge and generate high voltages for a variety of electrical applications.
Converting Potential to Kinetic Energy
Potential energy can be converted into kinetic energy, which is the energy of motion. This occurs when the forces acting on an object are unbalanced, causing the object to accelerate. Some examples of potential energy converting to kinetic energy include:
- A ball held at a height has gravitational potential energy due to the earth’s gravity acting on it. When dropped, this potential energy gets converted to kinetic energy as the ball accelerates towards the ground.
- A compressed spring has elastic potential energy. When released, the spring decompresses and pushes back, converting the potential energy into kinetic energy.
- Chemical potential energy stored in molecules gets released as kinetic energy during chemical reactions. For example, the potential energy stored in gasoline is converted into heat and kinetic energy when combusted in a car engine.
- Nuclear potential energy locked inside atomic nuclei gets converted into enormous amounts of kinetic energy during nuclear fission or fusion reactions.
The relationship between potential and kinetic energy is described by the law of conservation of energy. The total mechanical energy in a closed system remains constant – energy is never created or destroyed, just converted from one form to another. Understanding these energy conversions allows us to utilize potential energy to create useful kinetic energy for work and motion.
Potential Energy Diagrams
Potential energy diagrams, also known as potential energy curves, are graphs that show the potential energy of a system as a function of position or configuration. They are powerful visual tools that allow us to analyze the energetics of a system qualitatively and quantitatively.
On the x-axis of a potential energy diagram is the position or configuration of the system, which could be the distance between two atoms in a bond, the angle between two chemical bonds, or the relative orientation of molecules. The y-axis shows the potential energy of the system at each position/configuration.
Potential energy diagrams illustrate the stored energy present in stable arrangements like chemical bonds or molecular conformations. The minima on the graph represent lower energy, more stable configurations. Maxima represent higher energy, less stable transitional states. The deeper the well or lower the minimum, the more stable that configuration is.
The height of the energy barriers between minima gives the activation energy required to transition between different configurations. Overall, potential energy diagrams allow us to visualize the energetic landscape of a system and gain insights into kinetics, thermodynamics, and molecular structures.
Importance and Applications
Potential energy plays a crucial role in many aspects of our daily lives and modern technology. Here are some of the key ways that potential energy is important and applied:
Transportation – Vehicles, aircraft, and rockets utilize chemical potential energy in fuel to provide the kinetic energy of motion. Gravitational potential energy also allows gliders and downhill vehicles to convert height into speed.
Power Generation – Dams utilize gravitational potential energy of elevated water to drive electric generators as the water falls. Batteries and fuel cells convert chemical potential energy into electrical energy.
Industry and Manufacturing – Factories harness electrical potential energy to power motors and machinery. Compressed air and springs with elastic potential energy are used for specialized tools and applications.
Explosives – Gun powder, dynamite, and TNT contain great amounts of stored chemical potential energy that can be released rapidly in explosions for mining, demolition, and military uses.
Nuclear Power – The tremendous nuclear potential energy locked inside atomic nuclei can be tapped to generate electricity, drive naval vessels, or unfortunately, create weapons.
Renewable Energy – Sources like hydro, wind, and solar rely on gravitational, pressure, and chemical potential energy to produce clean power. Their intermittent nature highlights the importance of potential energy storage.
In short, the storage and conversion of potential energy enables modern civilization across transportation, electricity, manufacturing, and more. Mastering various forms of potential energy underpins technological and societal progress.
Summary
Potential energy is stored energy that an object has because of its position or chemical composition. The main types of potential energy include:
- Gravitational potential energy: Objects high above the ground have more gravitational potential energy than objects near the ground.
- Elastic potential energy: Stretched or compressed springs and other elastic materials store elastic potential energy.
- Chemical potential energy: The configuration of atoms and molecules in substances like food, fuel, and batteries contains chemical potential energy.
- Nuclear potential energy: The binding energy holding together atomic nuclei contains large amounts of potential energy.
- Electrostatic potential energy: Charged particles separated in space, like in batteries, have electrostatic potential energy.
Potential energy can be converted into kinetic energy, which is the energy of motion. A rock falling off a cliff converts gravitational potential energy into kinetic energy. Understanding potential energy helps explain how many mechanical and chemical processes work.