What Type Of Energy Increases With Temperature?
Thermal energy refers to the total kinetic energy of molecules within a substance, which is directly related to the temperature of that substance. Temperature measures the average kinetic energy of molecules and atoms, with higher temperatures indicating faster molecular motion. Thermal energy and temperature are intrinsically linked – as temperature increases, the average kinetic energy and thus thermal energy also increases. This relationship is fundamental to thermodynamics.
This article will provide an overview of how and why thermal energy rises with increasing temperature. We will explore real-world examples that demonstrate this relationship, as well as applications that rely on the connection between temperature and thermal energy.
Thermal Energy Basics
Thermal energy refers to the total kinetic energy of the atoms and molecules that make up a substance. This kinetic energy comes from the random motion and vibrations of the particles that comprise matter. The higher the temperature of a substance, the more active the atoms and molecules are, and thus the higher the thermal energy.
Temperature is a measure of the average kinetic energy of the particles in a substance. Temperature doesn’t directly quantify thermal energy, but rather how concentrated or spread out that energy is. Substances with the same temperature can have different thermal energies depending on the number of atoms/molecules present. But in general, higher temperature substances tend to have more total thermal energy.
Relationship Between Temperature and Thermal Energy
Thermal energy, also known as heat energy, is the energy associated with the motion and vibrations of atoms and molecules. As temperature increases, atoms and molecules gain kinetic energy and move faster and more vigorously. This increased molecular motion corresponds directly to an increase in thermal energy.
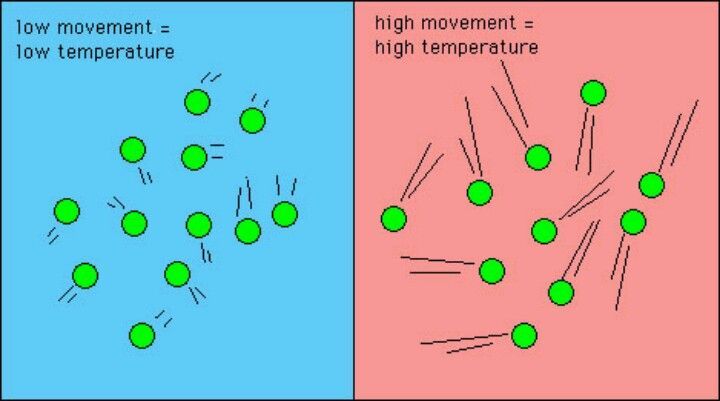
Temperature is a measure of the average kinetic energy of atoms and molecules in a substance. Thermal energy is directly proportional to temperature – as temperature increases, thermal energy also increases. This proportional relationship exists because temperature indicates the kinetic energy of individual atoms, while thermal energy refers to the total kinetic energy of all atoms in a substance.
To visualize this relationship, imagine a pot of water on the stove. As you increase the temperature by turning up the stove, the water molecules absorb energy, move faster, and collide more frequently. The higher kinetic energy of the faster moving molecules corresponds to an increase in thermal energy of the water. So heating the water increases its temperature, which in turn increases its thermal energy.
Increasing Temperature Increases Thermal Energy
There is a direct correlation between temperature and thermal energy. Thermal energy refers to the total kinetic energy of all the molecules within a substance. This kinetic energy is tied to the motion and vibrations of the molecules. As more heat is added to a substance, the molecules gain more kinetic energy and start moving faster and vibrating more intensely. This increased molecular motion corresponds directly to an increase in temperature.
Likewise, removing heat causes the molecules in a substance to move more slowly and vibrate less. With less kinetic energy, the temperature decreases. So heating increases thermal energy which raises the temperature, while cooling decreases thermal energy which lowers the temperature. This relationship allows temperature measurements to be an indicator of the thermal energy within a substance. The higher the temperature, the more thermal energy the molecules possess.
Examples
There are many everyday examples where increasing the temperature results in an increase in thermal energy:
Heating water on a stove increases its thermal energy. As the water absorbs heat from the stove burner, kinetic energy at the molecular level increases, resulting in greater molecular motion and collisions. This manifests as increasing water temperature. The hotter the water becomes, the more thermal energy it contains.
Compressing gas in a bicycle pump increases its thermal energy. When a gas is compressed into a smaller volume, the molecules are forced closer together. The increased molecular collisions and kinetic energy is detected as a rise in temperature. The more the gas is compressed, the more thermal energy it gains.
Thermal Energy Flows from High to Low Temperature
According to the second law of thermodynamics, heat flows spontaneously from higher temperature objects to lower temperature objects. This means that thermal energy transfers from high energy molecules to low energy molecules during molecular collisions.
For example, when a pot of water is heated on the stove, the thermal energy from the stove burner (high temperature) flows into the water (lower temperature), increasing the water’s thermal energy and thus its temperature. The water will never spontaneously transfer thermal energy back to the burner to make it hotter.
This principle explains why an ice cube melts when placed in a warm room. The higher thermal energy in the room air molecules colliding with the ice cube cause thermal energy to flow into the ice, raising its temperature until it melts.
The directional flow of thermal energy from hot to cold is why an ice cube cools a drink – the thermal energy flows from the warmer drink into the colder ice cube.
Understanding how thermal energy spontaneously diffuses from higher temperature matter to lower temperature matter explains many everyday phenomena and has useful applications in science and engineering.
Practical Applications
Many everyday devices and systems rely on the principle that thermal energy increases with temperature. Three prime examples are:
- Car engines – Gasoline ignites in the cylinders to generate heat and increase thermal energy, pushing the pistons and powering the car.
- Nuclear reactors – Fission reactions generate intense heat to boil water for steam turbines and create electricity.
- Human metabolism – Our cells perform chemical reactions that convert food into thermal energy to maintain body temperature.
These examples demonstrate how harnessing greater thermal energy from higher temperatures enables mechanical motion and electrical power generation across industries and biology.
Interesting Facts
Two interesting real-world examples that demonstrate how higher temperature leads to greater thermal energy are hot air balloons and reptiles/amphibians.
Hot air balloons rely on the principle that hotter air has more thermal energy, and thus more buoyancy. The air inside the balloon is heated, usually by propane burners aimed into the envelope. This makes the air less dense than the surrounding cooler air, allowing the balloon to rise. The pilots can control the balloon’s altitude by increasing or decreasing the temperature of the air inside.
Cold-blooded animals like frogs and reptiles also take advantage of warmer air’s higher thermal energy. By positioning their bodies in sunny spots, they can absorb heat from the sun’s rays. This increases their body temperature and metabolic rate, allowing them to become more energetic and active. It’s why you often see these animals basking on warm rocks or sand.
Limitations
While it’s generally true that thermal energy increases with temperature, there are some limitations to this relationship.
At extremely high temperatures, molecules can break down and thermal energy actually decreases. Once temperatures reach thousands or millions of degrees, individual atoms within molecules have so much energy that the molecules themselves can split apart in a process called dissociation. The breaking of molecular bonds requires energy, so as more and more molecules dissociate at higher temperatures, less thermal energy is available in the system.
Additionally, quantum effects can decouple temperature from thermal energy at very low temperatures near absolute zero. As materials approach zero kelvin, the discrete energy levels of quantum systems start to become apparent, and adding more thermal energy does not always increase the temperature. For example, the motions of electrons in a metal are quantized at low temperatures, limiting their ability to gain kinetic energy from added heat.
So while increasing temperature generally increases thermal energy, at extreme high and low temperature ranges, the relationship breaks down due to dissociation of molecules or quantum effects.
Conclusion
In summary, thermal energy and temperature are intrinsically linked in physics. As temperature increases, the thermal energy of a substance also increases as the molecules gain kinetic energy. We see examples of this in real world applications like engines and chemical reactions. While there are some exceptions like during phase changes, in general, higher temperatures result in higher thermal energy which can be transferred or do work. Though simple on the surface, fully understanding the nuances of this relationship has enabled many advances in science and technology.