What Type Of Energy Do All Objects Have?
Energy is the capacity to do work or produce heat. All objects, from the tiniest particles to the largest stars, contain energy in one form or another. The goal of this article is to explore the different types of energy that all objects possess, including kinetic, potential, nuclear, thermal, electromagnetic, and sound energy. We will examine how energy can convert from one form to another, and also touch on the quantum physics perspective of energy.
Kinetic Energy
Kinetic energy refers to the energy an object has due to the motion of its particles. The faster an object moves, the more kinetic energy it possesses. Kinetic energy depends on both the mass and the velocity of an object.
For example, a car moving at 60 mph has more kinetic energy than the same car moving at 30 mph. This is because kinetic energy increases exponentially with velocity. Doubling the velocity of an object quadruples its kinetic energy. Kinetic energy also depends on mass – a car has much more kinetic energy than a bicycle moving at the same speed.
Kinetic energy can be transferred between objects. For instance, when a moving billiard ball strikes a stationary one, it transfers some of its kinetic energy to start the stationary ball moving. The kinetic energy of the initially moving ball decreases while the kinetic energy of the second ball increases.
Other examples of kinetic energy include a soccer ball being kicked, a gust of wind blowing leaves, and waves crashing on a shore. Anything in motion possesses kinetic energy proportional to its mass and the square of its velocity.
Potential Energy
Potential energy is the stored energy an object has due to its position or composition. There are different types of potential energy:
- Gravitational Potential Energy – This depends on an object’s height above the ground, due to the pull of gravity. The higher an object is positioned, the more gravitational potential energy it has. For example, a book held 2 meters above the ground has more gravitational potential energy than when it is sitting on a table.
- Elastic Potential Energy – This is stored in elastic materials that are stretched or compressed. For example, a stretched rubber band has more elastic potential energy than a relaxed rubber band.
- Chemical Potential Energy – This is energy stored in the bonds between atoms and molecules. Chemical potential energy can be released in chemical reactions when the molecular bonds are broken and rearranged.
The key factor for potential energy is that it depends on an object’s position or composition. For example, holding an object at a height gives it gravitational potential energy that can be released if it falls. The composition of molecules and chemical bonds leads to potential energy that can be released in chemical reactions. Potential energy is converted into kinetic energy when an object’s position changes or chemical bonds are rearranged.
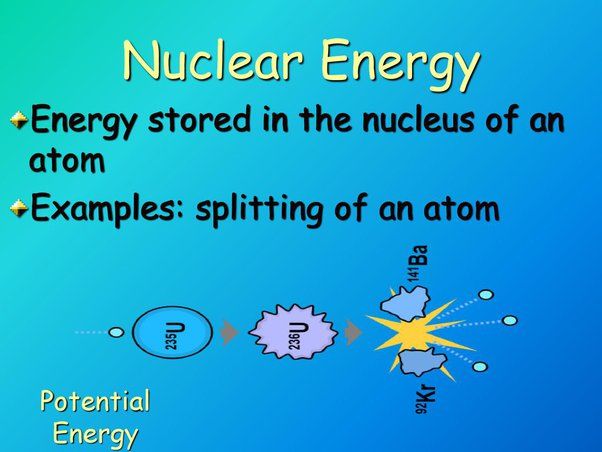
Nuclear Energy
Nuclear energy refers to the energy stored in the nucleus of an atom. According to Albert Einstein’s theory of relativity, energy and mass are interchangeable. This means that tiny amounts of mass can be converted into enormous amounts of energy. This is known as mass-energy equivalence and is represented by Einstein’s famous equation E=mc2, where E is energy, m is mass, and c is the speed of light.
Nuclear energy comes from binding energy, which is the energy required to keep the protons and neutrons of an atom’s nucleus together. The binding energy of the nucleus is always smaller than the sum of the individual masses of the constituents, meaning that some mass is lost during nucleus formation. This lost mass is converted into nuclear binding energy according to Einstein’s mass-energy equivalence.
There are two main examples of utilizing nuclear energy – nuclear fission and nuclear fusion.
Nuclear fission involves splitting the nucleus of a heavy element like uranium or plutonium into two lighter nuclei, which releases energy. This process also produces neutrons that can trigger a chain reaction by splitting further uranium nuclei, releasing more energy.
Nuclear fusion takes place when two light atomic nuclei fuse together to form a heavier nucleus, converting some of the mass into energy. Fusion reactions power the sun and other stars. Scientists are researching ways to control fusion reactions for energy production on Earth.
Both fission and fusion reactions release enormous amounts of nuclear energy from tiny amounts of matter, demonstrating the incredible power that can be unlocked from the atomic nucleus.
Thermal Energy
Thermal energy refers to the total kinetic energy of all the atoms and molecules that make up an object or substance. It is directly related to the temperature of the object or substance. The higher the temperature, the more thermal energy it contains, as the atoms and molecules vibrate and move faster.
Thermal energy can be transferred from one object or substance to another in three main ways:
Conduction is the transfer of thermal energy through direct contact between objects, without any motion of the objects themselves. For example, when you place a spoon in a hot cup of coffee, the metal spoon conducts heat from the coffee to the spoon, causing the spoon to feel warm. The hotter molecules in the coffee collide with the cooler spoon molecules, transferring kinetic energy.
Convection is the transfer of thermal energy by the movement of heated fluid or gas. For example, as air is heated it becomes less dense and rises. Cooler, denser air then moves in to take its place, creating convection currents. This allows heat to be distributed through the entire space. Convection currents are responsible for the motion of air in the atmosphere and water in the oceans and lakes.
Radiation is the transfer of thermal energy via electromagnetic waves, like infrared radiation. All objects emit electromagnetic radiation depending on their temperature. For example, a hot stovetop emits infrared radiation that you feel as heat when you hold your hand above it. Radiation does not require direct contact between objects, it can travel through space.
Electromagnetic Energy
Electromagnetic energy is a form of energy that is associated with electromagnetic fields and waves. It is the energy carried by photons, which are particles of light. Electromagnetic energy includes visible light such as the light we see, as well as other forms of radiation that are invisible to the human eye including ultraviolet light, infrared radiation, radio waves, and X-rays.
Electromagnetic energy is generated when an electric charge accelerates or decelerates. The oscillations and rotations of charged particles such as electrons and protons give rise to electromagnetic waves that travel through space at the speed of light. These waves carry energy away from their source in the form of electric and magnetic fields.
Examples of electromagnetic energy include:
- Visible light – The visible spectrum that humans can see such as the colors red, orange, yellow, green, blue, indigo and violet.
- Microwaves – Used for radar, cooking, and telecommunication networks. Microwaves have longer wavelengths than visible light.
- X-rays – Can pass through soft tissues of the body and are used to take images of bones and teeth. X-rays have shorter wavelengths and higher frequencies than visible light.
- Gamma rays – The most energetic form of electromagnetic waves. Produced by radioactive decay in the universe.
- Radio waves – Used for radio communication, broadcasting, radar, and other navigation systems. Have very long wavelengths but carry very low energy.
All electromagnetic energy travels at the speed of light in a vacuum. The different categories relate to different frequency and wavelength bands. Although we cannot see many types of electromagnetic radiation, they make up the full spectrum of electromagnetic energy.
Sound Energy
Sound energy is a form of kinetic energy associated with the motion of atoms and molecules in matter. When an object vibrates or oscillates, it creates a mechanical wave that propagates through a medium like air or water. This wave transports energy from one location to another without actually transporting any matter.
As the object continues to vibrate, it causes the particles in the surrounding medium to vibrate as well. These particles bump into neighboring particles, transferring the energy along. The particles themselves do not travel far, but the wave energy gets passed along – hence the term “wave”.
The energy transported through the medium by the wave is sound energy. The molecules and atoms vibrate parallel to the direction of the wave. As the wave passes through the medium, it causes regions of compression and rarefaction. In compression regions, the molecules and atoms are pushed close together. In rarefaction regions, they are spread apart. These vibrational disturbances carry sound energy without transporting matter.
The frequency of the sound determines the pitch. A higher frequency leads to a higher pitched sound. The greater the amplitude of the wave, the louder the sound. As sound waves move through matter, some of the energy gets converted to thermal energy through friction and viscosity. This leads to a diminishing loudness of the sound as it travels farther from the source.
Interconversions Between Types
Energy is always conserved, meaning it cannot be created or destroyed, only converted between different forms. Here are some examples of how energy can change from one type to another:
Kinetic to Thermal: When you rub your hands together, the kinetic energy of the motion converts into thermal energy in the form of heat.
Potential to Kinetic: When a rollercoaster goes down a steep incline, the potential energy from the height converts into kinetic energy of motion.
Chemical to Thermal: When wood burns, the chemical energy stored in the molecules releases as thermal energy in the form of fire.
Nuclear to Thermal: In nuclear power plants, nuclear energy from uranium atoms converts into thermal energy which is used to boil water into steam to spin turbines.
Thermal to Mechanical: In car engines, the thermal energy from combustion converts into mechanical energy to move the pistons.
Energy conversions happen continuously all around us. The law of conservation of energy states that the total energy in an isolated system always remains constant. Energy is never lost, just changed from one form into another.
Quantum Physics Perspective
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. A key principle of quantum theory is that energy can only exist in discrete amounts, or quanta. This quantization of energy applies to all systems, including electromagnetic radiation, vibrations, rotations, and the energy levels within atoms and molecules.
According to quantum physics, it is impossible to accurately determine both the position and momentum of a particle at the same time. This uncertainty principle implies that even a vacuum contains transient electromagnetic waves and particles that pop into and out of existence. Furthermore, particles can exhibit the properties of both waves and particles. Rather than distinct types, the various forms of energy are different manifestations of the same underlying quantum fields.
The quantization and wave-particle duality of energy at microscopic scales has proven that the classical view of energy as solely kinetic, potential and different distinct types is incomplete. Quantum theory reveals that energy is an innate fundamental property of all quantum fields and particles. The quantum perspective unifies the different forms of energy and provides a deeper understanding of the intrinsic nature of energy in all matter.
Conclusion
Energy is a fundamental property of all objects in our universe. As we have explored, there are many different types of energy that objects can possess depending on their motion, position, temperature, and composition.
Kinetic energy arises from an object’s motion, while potential energy relates to an object’s position or composition. Thermal energy is associated with the kinetic energy of atoms and molecules, while nuclear energy exists in the nuclei of atoms. Electromagnetic energy includes forms like light, radio waves, and x-rays. Sound energy is the energy of vibrating molecules transmitted as waves. These different forms of energy can convert into one another under the right conditions.
Quantum physics provides an even deeper understanding of energy as related to the behavior of subatomic particles. At the most fundamental level, energy is woven into the fabric of our universe. By studying energy, we gain insight into the nature of motion, heat, light, and the forces that govern our world. An appreciation for the many manifestations of energy is key to understanding our natural and technological world.