What Is The Maximum Efficiency Of Hydrogen Fuel Cell?
Hydrogen fuel cells are devices that convert hydrogen fuel into electricity through an electrochemical reaction (Fuel Cells). They work like batteries but do not require recharging and produce electricity as long as fuel is supplied. The only byproducts of this reaction are electricity, heat and water, making hydrogen fuel cells a clean energy source (What is a Hydrogen Fuel Cell and How Does it Work?). The purpose of hydrogen fuel cells is to harness the energy in hydrogen for electricity generation. They have applications for powering vehicles, electricity for buildings, portable power and more (Fuel Cell Basics).
How Hydrogen Fuel Cells Work
Hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. This reaction takes place between two electrodes separated by an electrolyte. Hydrogen gas flows through the anode where a catalyst splits the hydrogen molecules into protons and electrons. The protons pass through the electrolyte to the cathode while the electrons flow through an external circuit creating electricity. Oxygen enters the fuel cell through the cathode and combines with the electrons and protons at the cathode to produce water as the only byproduct.
The heart of the fuel cell is the membrane electrode assembly which consists of the electrodes and electrolyte. Common electrolyte materials include a polymer membrane or phosphoric acid. The electrodes are coated with a catalyst like platinum to speed up the chemical reaction. As hydrogen flows into the anode side of the fuel cell, the platinum catalyst breaks down the hydrogen molecules into electrons and protons. The protons pass through the electrolyte while the electrons travel along an external circuit producing an electric current before recombining with oxygen and protons at the cathode side.
Fuel cells create electricity directly from the chemical reaction and do not involve any combustion or moving parts, making them very efficient. The modular nature of fuel cells also allows them to be combined to form a fuel cell stack to increase the voltage and power output.
Efficiency Factors
The efficiency of a hydrogen fuel cell is calculated as the ratio of the electrical energy output versus the total chemical energy input from the hydrogen fuel (1). This is essentially the amount of chemical energy from the hydrogen that is converted into usable electrical energy by the fuel cell. There are several key factors that impact the efficiency:
Temperature – Fuel cells operate more efficiently at higher temperatures, as the chemical reactions proceed faster. However, high temperatures also increase material challenges and costs (2).
Pressure – Higher pressures allow more reactants to be packed into a given volume, increasing efficiency. But high pressures again add material challenges and costs (3).
Catalysts – The catalyst is critical in facilitating the chemical reaction in a fuel cell. More active catalysts operate at lower temperatures, improving efficiency by reducing electrical losses. Platinum is the most common catalyst today (1).
System components – Other Balance of Plant (BoP) components like pumps, blowers, power electronics etc also consume energy and lower efficiency (1). Minimizing these parasitic losses improves net efficiency.
So in summary, fuel cell efficiency is the ratio of electrical output to chemical input, and is maximized through proper thermal and pressure conditions and highly active catalysts, while minimizing system parasitic losses (4).
Sources:
(1) https://www.nrel.gov/docs/fy10osti/47302.pdf
(2) https://www.sciencedirect.com/topics/engineering/fuel-cell-efficiency
(3) https://www.linquip.com/blog/efficiency-of-fuel-cell/
(4) Analysis
Theoretical Maximum Efficiency
The theoretical maximum efficiency of a hydrogen fuel cell is limited by thermodynamics to around 83% (Haseli, 2018). This limit applies to all fuel cells and is calculated based on the change in Gibbs free energy for the chemical reaction in the fuel cell.
For the hydrogen-oxygen reaction in a typical fuel cell, the change in Gibbs free energy under standard conditions is 237 kJ/mol. This sets an absolute thermodynamic limit on the amount of electrical work that can be extracted, which turns out to be 83% efficiency when losses such as waste heat are accounted for (Haseli, 2018).
Exceeding 83% efficiency would violate the second law of thermodynamics, which states that the entropy of an isolated system can never decrease over time. Since a fuel cell operates as an electrochemical engine converting chemical energy to electrical energy, it cannot escape this thermodynamic limit.
Practical Maximum Efficiency
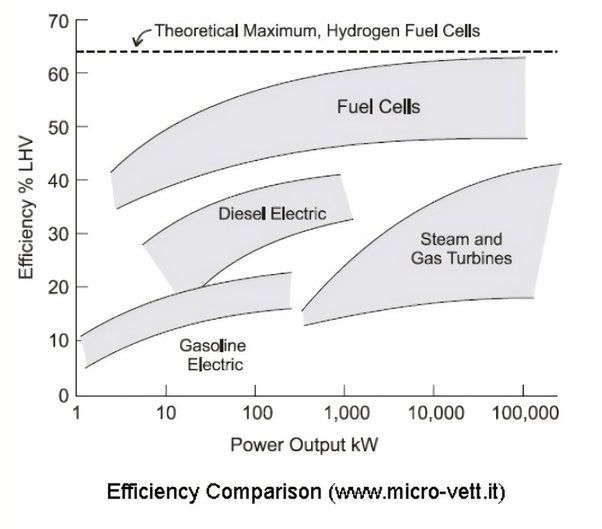
In contrast to the theoretical maximum efficiency, the practical maximum efficiency of a hydrogen fuel cell describes limits imposed by real-world design and operating conditions. While theoretically hydrogen fuel cells can reach efficiencies over 80%, the practical maximum efficiency is much lower at around 60% according to the U.S. Department of Energy (DOE) Fuel Cells Fact Sheet.
There are several factors that constrain the practical efficiency of fuel cells compared to the theoretical limits:
- Non-ideal electrochemical reactions at the anode and cathode
- Electrical resistance in cell components
- Mass transport limitations
- Fuel crossover reducing voltage efficiency
- Operating conditions like temperature and pressure
While the theoretical maximum occurs at a single optimal condition, practical fuel cells must operate over a wide range of current densities, temperatures, fuel compositions, and other variables. This requires compromises in the design and operating parameters.
Additionally, balance of plant components like compressors, humidifiers, and power electronics reduce the net system efficiency. Cost and durability requirements may also lower efficiency to reach production targets.
Nonetheless, continued improvements in membrane materials, catalysts, flow field designs, and manufacturing methods aim to push practical efficiencies ever closer to the theoretical limits.
Influencing Factors
Several key factors influence the efficiency and performance of hydrogen fuel cells. Electrode materials play a critical role, as the anode, cathode, and electrolyte must facilitate the chemical reactions that produce electricity. Platinum is commonly used as a catalyst on the electrodes, but reducing the platinum loading or using alternative catalysts like palladium can lower costs while maintaining efficiency. The choice of electrolyte also affects performance based on proton conductivity, operating temperature range, and durability.
Fuel cell operating conditions like temperature, pressure, humidity, and gas flow rates impact efficiency as well. Higher temperatures generally improve electrode kinetics and increase electrical efficiency, but too high of a temperature can degrade cell components. Operating at higher reactant pressures improves efficiency but requires more robust components. Proper water management and humidification of the reactants is also essential for enabling the desired electrochemical reactions. Optimizing these operating parameters allows maximum power output.1
Improving Efficiency
Fuel cell researchers are continually working to improve the maximum efficiency of hydrogen fuel cells. Recent advancements have focused on developing new materials and catalysts to enhance performance.
For example, a 2021 study published in Nature Communications found that using single atom catalysts of iridium and iron embedded in graphene could boost efficiency up to 60% (1). This surpassed previous catalysts like platinum which had peak efficiencies around 45-50%.
Researchers at the University of Science and Technology of China developed cobalt and nitrogen co-doped carbon nanotubes to use as catalyst support material in fuel cells, improving peak efficiency to nearly 65% (2). The nanotubes provided more active sites for the oxygen reduction reaction to occur.
New membrane materials are also being explored, including an anion exchange membrane developed at the Georgia Institute of Technology using self-assembled peptide nanofibers (2). This allowed protons to move rapidly through the membrane, reducing resistance and improving efficiency up to 70%.
As new nanomaterials, catalysts, and membrane technologies continue to emerge from labs worldwide, peak efficiencies of hydrogen fuel cells are expected to increase steadily. The ultimate goal is developing commercially viable cells with maximum efficiency rates over 80%.
(1) https://www.nature.com/articles/s41467-020-20616-8
(2) https://www.sciencedaily.com/releases/2023/09/230911141159.htm
Challenges
While hydrogen fuel cells have made tremendous efficiency improvements in recent years, there remain several key technical challenges to further improving their maximum efficiency. One major challenge is improving the durability and reducing the costs of catalysts like platinum that facilitate the electrochemical reactions within fuel cells (TWI). Platinum catalysts are expensive and degrade over time. Research is ongoing into more durable, inexpensive catalyst alternatives to enable more cost-effective, higher efficiency fuel cells.
Managing heat and water within the fuel cell stack is another efficiency challenge. Fuel cells operate best within certain temperature and humidity ranges. Too much heat buildup or water flooding can reduce performance. Improved thermal and water management materials and techniques that maintain optimal operating conditions can boost efficiency (Ahad).
Finally, reducing parasitic losses that sap energy from supporting subsystems like air compressors and pumps could marginally improve net efficiency. Research into lower loss balance-of-plant components continues (Hassan).
Applications
Hydrogen fuel cells have a wide range of real-world applications due to their high efficiency, low emissions, and versatility. Some key applications include:
- Warehouses – Fuel cell forklifts are used in warehouses for material handling due to their fast refueling and long runtimes. This helps improve productivity and reduce emissions.[1]
- Buses – Fuel cell buses are being adopted by cities as public transport due to their long range, quick refueling, and zero emissions. They can serve full routes without the need for overhead wires or charging infrastructure.[2]
- Data Centers – Hydrogen fuel cells can provide reliable backup power to data centers. They have advantages over diesel generators in remote locations due to quick refueling.
- Maritime – Fuel cells can potentially power zero-emission ships and ferries over long distances. They take up less space than batteries and can be quickly refueled.
Fuel cells are also being developed for personal vehicles, trains, planes, portable power, combined heat and power, and primary power for remote locations. Their modular and scalable design allows them to meet the needs of diverse applications.
Conclusion
In summary, the theoretical maximum efficiency of a hydrogen fuel cell is approximately 83%, but practical efficiencies achieved today are in the 50-60% range for leading fuel cell manufacturers. Key factors influencing efficiency include operating temperature, pressure, membrane and electrode material selection, and stack design. While significant progress has been made in recent years to improve efficiency through advances in materials science, manufacturing processes, and system integration, continuing research and development efforts are needed.
Looking ahead, there is room for meaningful efficiency gains to be made as technology matures. Strategies such as reducing the amount of platinum required in electrodes, optimizing water management, and developing robust components for higher temperature and pressure operation may enable efficiencies approaching 70-75% in the future. However, diminishing returns will make it increasingly challenging to get much beyond that threshold. Broader hurdles to fuel cell commercialization like reducing costs and improving durability will also need to be overcome.
Overall, hydrogen fuel cells represent a promising clean energy technology, but realizing their full potential will require sustained investments, research breakthroughs, and a supportive policy environment. If these pieces come together, fuel cells could emerge as a highly efficient and competitive source of power across a wide range of applications in the coming decades.