What Is The Energy Stored In The Bonds Of Matter?
The energy stored in the bonds of matter refers to the bond energy, which is the amount of energy required to break a chemical bond within a molecule. This energy is released when the bond is formed from the separated atoms.
Understanding bond energies is crucial for explaining the stability of molecules, predicting the direction of chemical reactions, and calculating reaction energies. The more energy stored within chemical bonds, the more stable the molecule. Breaking bonds requires energy input, while forming bonds releases energy.
Chemical Bonds
Chemical bonds are what hold atoms together in molecules and compounds. There are different types of chemical bonds that form depending on the types of atoms involved and their properties.
The main types of chemical bonds are:
- Ionic bonds – Form between a metal and a nonmetal atom. The metal loses electrons to become a positive ion, while the nonmetal gains those electrons to become a negative ion. The opposite charges attract each other to form the bond.
- Covalent bonds – Form when atoms share electron pairs between each other. Each atom contributes one or more electrons to a shared electron pair that connects the atoms. Covalent bonds can be polar or nonpolar depending on whether the shared electron pair is equally shared between the atoms.
- Metallic bonds – Form in metals between positive metal ions arranged in a lattice structure and a “sea” of delocalized electrons that are free to move throughout the structure. The attraction between the free-moving electrons and positive metal ions holds the metallic bond together.
The type of chemical bond that forms has important implications for the properties of the compound, such as melting point, conductivity, solubility, and more. The strength of chemical bonds also determines how much energy is required to break the bonds during chemical reactions.
Bond Energy
Bond energy, also known as bond dissociation energy, is the amount of energy required to break a chemical bond between two atoms in a gaseous molecule. It is measured in kilojoules per mole (kJ/mol).
The bond energy represents the strength of a chemical bond. Stronger chemical bonds require more energy to break and therefore have higher bond energies. For example, a triple bond between two carbon atoms has a bond energy of 839 kJ/mol, while a single bond between the same two carbons has a bond energy of only 347 kJ/mol.
When a bond breaks, the atoms must move far enough apart so that the remnant molecular fragments can no longer interact. Overcoming the attractive forces that hold the atoms together requires an input of energy, which comes from the absorption of heat or light. The amount of energy absorbed when a mole of bonds are broken is defined as the bond energy.
In summary, bond energy quantifies the strength of a chemical bond and reflects the energy needed to break that bond homolytically. Stronger bonds have higher bond energies.
Measuring Bond Energies
There are several methods used to measure bond energies in chemistry:
Bond Dissociation Energy
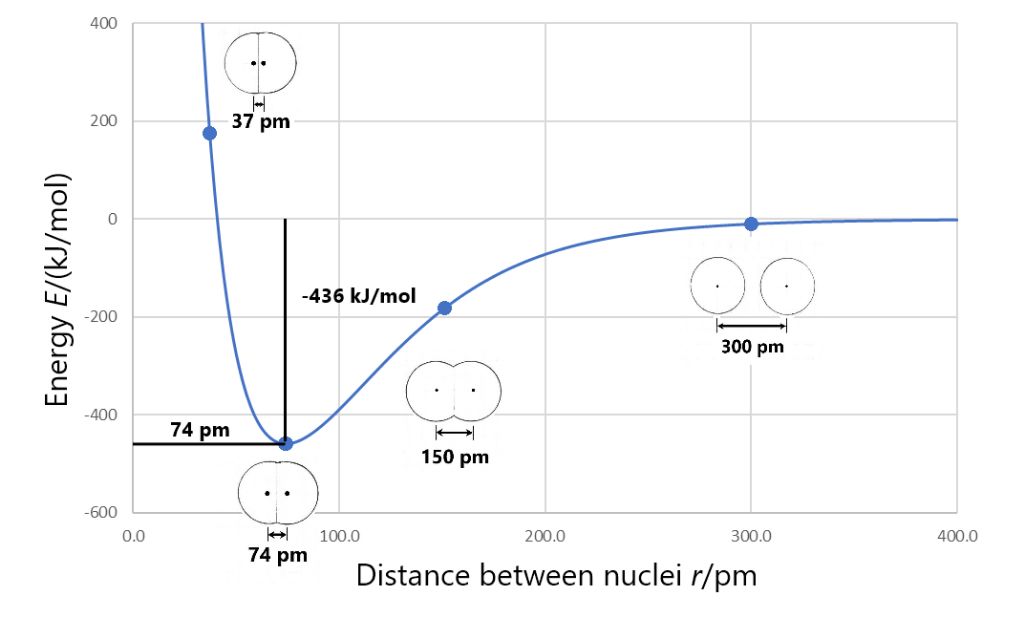
This involves breaking a bond and measuring the energy required. For example, breaking a H-H bond requires 432 kJ/mol of energy. The bond dissociation energy for H-H is therefore 432 kJ/mol. This method can directly measure bond energies.
Reaction Enthalpies
This uses Hess’s law to measure the enthalpy change of a reaction, which can be related to the bond energies of the reactants and products. By calculating multiple reaction enthalpies, the individual bond energies can be derived. This provides an indirect method to determine bond energies.
Computational Methods
Advanced computational methods like density functional theory can calculate bond dissociation energies through modeling. This provides a theoretical approach to estimate bond energies.
Spectroscopic Methods
Techniques like infrared spectroscopy can measure bond vibration frequencies, which relate to bond force constants and bond dissociation energies. This experimental approach also allows estimation of bond energies.
Factors Affecting Bond Energy
There are several factors that determine and influence the strength of a chemical bond between atoms and thus the bond energy. Some key factors include:
Electronegativity
Electronegativity refers to the tendency of an atom to attract shared electrons towards itself. Atoms with higher electronegativity form stronger bonds as the shared electrons are closer. As electronegativity difference between atoms increases, the bond strength and bond energy decreases.
Bond Length
Bond length refers to the distance between the nuclei of two bonded atoms. In general, shorter bond lengths correlate with stronger bonds and higher bond energies, as there is greater electrostatic attraction between the bonded nuclei. Longer bond lengths tend to have lower bond energies.
Hybridization
Hybrid orbitals tend to form stronger, more stable bonds compared to pure or unhybridized orbitals. This is because hybrid orbitals allow better orbital overlap. Greater overlap leads to increased bond strength and higher bond energy.
Resonance
Resonance stabilization helps strengthen bonds by allowing delocalization of electrons across multiple bonds. Resonance leads to greater stability and thus higher bond energies for the resonating bonds.
Applications
Bond energies have important practical applications in many scientific fields and industries.
Examples of using bond energy
Bond energies can help predict and explain:
- Reaction rates in chemical reactions
- How much energy is released or absorbed during chemical reactions
- The stability of molecules
- How easy it is to break chemical bonds
Some examples of using bond energy calculations include:
- Estimating the energy released during combustion reactions like burning fossil fuels
- Predicting the speed of reactions for industrial chemical production
- Determining reaction mechanisms and favorable pathways
- Explaining why some molecules are more reactive than others
- Modeling explosive reactions and energetics
- Analyzing metabolic pathways and energy transfers in biological systems
Bond energies provide insights into real-world chemistry and are a useful tool across many fields and applications.
Bond Energy and Stability
There is a direct relationship between bond energy and the stability of a chemical bond. Bonds with higher bond energies tend to be more stable and difficult to break. This is because more energy is required to break bonds with higher bond energies.
For example, a carbon-carbon triple bond has a higher bond energy than a carbon-carbon single bond. The carbon-carbon triple bond requires 940 kJ/mol of energy to break, while the carbon-carbon single bond only requires 347 kJ/mol. This indicates the triple bond is more stable and difficult to break than the single bond.
In general, multiple bonds (double and triple bonds) have higher bond energies than single bonds between the same atoms. This greater bond energy corresponds to greater stability of the multiple bond. Multiple bonds contain more shared electron pairs between the atoms, resulting in stronger bonding and stability.
Bond energy also correlates with bond length. Bonds with higher bond energies tend to have shorter bond lengths. Shorter bond lengths indicate greater bond strength and stability. The shorter distance allows for greater overlap of electron orbitals and stronger covalent bonding between the atoms.
In summary, higher bond energy equates to greater chemical bond stability. Bonds with high energies require more energy input to destabilize and break the bond. They tend to have shorter bond lengths as well. Understanding the stability provided by bond energies helps predict chemical properties and reactivity.
Bond Energy and Reactivity
The bond energy is directly related to the reactivity of a molecule. Molecules with strong bonds that require a lot of energy to break are less reactive. On the other hand, molecules with weak bonds that can easily break are more reactive.
For example, diatomic nitrogen (N2) has a very strong triple bond with a bond energy of 945 kJ/mol. This makes nitrogen gas very stable and unreactive under normal conditions. To break the strong triple bond and get nitrogen to react requires adding a lot of energy, such as high temperatures or catalysts.
In contrast, hydrogen gas (H2) has a much weaker bond energy of 432 kJ/mol. The H-H bond can be broken relatively easily, so hydrogen readily reacts with other molecules. Less energy input is required to get hydrogen to react.
When thinking about chemical reactivity, the rule of thumb is:
The stronger the bond, the lower the reactivity.
The weaker the bond, the higher the reactivity.
Therefore, bond energy values provide useful information about how readily a molecule can participate in chemical reactions. This concept has many applications in chemistry, such as predicting reaction mechanisms, product ratios, and more.
Bond Dissociation Energy
Bond dissociation energy (BDE) is the amount of energy required to break a chemical bond and form neutral isolated atoms. It is often used as a measure of the stability of a bond. A bond with a high bond dissociation energy requires more energy to break and is considered more stable.
Bond dissociation energy is usually expressed in units of kilojoules per mole (kJ/mol). It can be measured experimentally using techniques like photolysis or reaction calorimetry. BDE values provide useful information about how reactive a molecule is and the strength of its bonds.
In general, bonds that are shorter and between atoms with higher electronegativity have higher bond dissociation energies. Double and triple bonds also tend to have higher bond dissociation energies than single bonds. The surrounding chemical environment can also affect bond dissociation energy.
Knowing the bond dissociation energy is helpful for predicting the feasibility and energy balance of chemical reactions. Bonds with lower bond dissociation energies tend to more easily undergo homolysis (bond dissociation). Weaker bonds break more readily in chemical reactions.
Conclusion
In conclusion, the energy stored in the bonds of matter is known as bond energy. Bond energy is the amount of energy required to break a chemical bond in a molecule. It represents the strength and stability of a bond. Measuring the bond energies of different bonds provides chemists with valuable information about the properties and reactivity of molecules.
The strength and stability of chemical bonds is hugely important in chemistry. Strong, stable bonds hold molecules together, while bonds that are easily broken allow molecules to react and form new substances. The bond dissociation energy directly correlates to bond strength – molecules with strong bonds that require lots of energy to break tend to be very stable.
Knowing the bond energies of molecules helps predict chemical reactions. Bonds that have lower bond energy will more readily break and react with other molecules. Weak bonds break more easily, allowing new bonds to form. Understanding bond strengths allows chemists to better control chemical reactions.
Overall, the concept of bond energy provides critical insights into the forces that hold matter together at the molecular level. By measuring bond energies, chemists gain knowledge that helps explain the properties and behaviors of different substances.