What Is The Effect Of Heat On Matter?
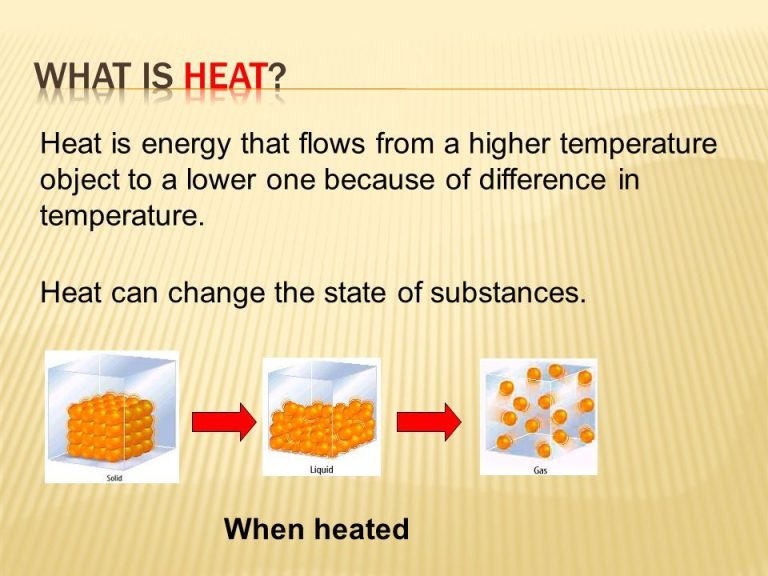
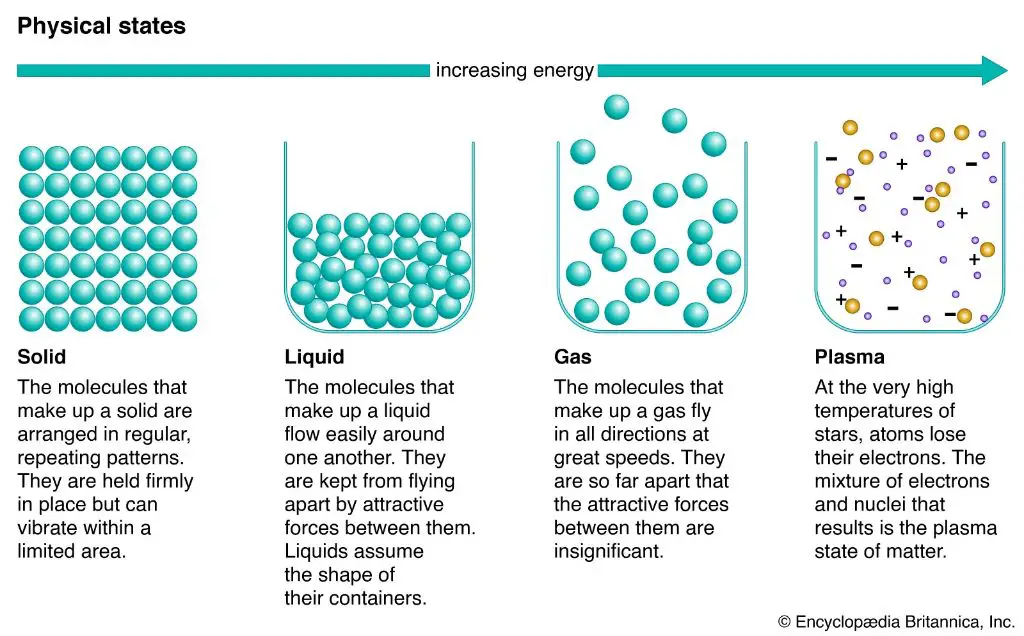
Heat is the transfer of thermal energy, while matter refers to anything that has mass and takes up space. When heat is applied to matter, it causes the atoms and molecules that make up that matter to move faster and spread apart. This can lead to various effects such as expansion, state changes, and chemical reactions. The three common states of matter are solids, liquids, and gases. Solids have a defined shape and volume. Liquids have a defined volume but change shape based on their container. Gases have no defined shape or volume.
Thermal Expansion
When matter is heated, the atoms and molecules gain kinetic energy and start moving faster and vibrating more. Since the atoms take up more space when vibrating faster, this causes the matter to expand in size. Solids, liquids and gases all exhibit thermal expansion when heated up.
On the atomic level, heating increases the vibrational kinetic energy of the atoms. As the atoms vibrate more rapidly within the molecular structure, the distances between the atoms increase slightly. This causes each molecule to take up a bit more space, which adds up to a measurable expansion in the overall size of the material.
The amount of expansion depends on the material and the temperature change. Solids usually expand the least, while gases have the greatest expansion. However, the expansion is proportional to the temperature change for a particular material. Higher temperature increases lead to greater expansion.
State Changes
When matter is heated, it can undergo state changes from solid to liquid to gas. This occurs because heating increases the kinetic energy and vibrational motion of the molecules in the matter. At certain temperatures, the molecules have enough energy to break free of the rigid structure of a solid and enter the more free-flowing structure of a liquid or gas.
In a solid, the molecules are tightly packed in an orderly lattice structure and cannot move freely. As heat is applied, the vibrations of the molecules increase until enough energy is added for the molecules to break free of their fixed positions and slide past each other. This allows the matter to take on the properties of a liquid, which can flow and change shape but still has a defined volume. If more heat is continually added, eventually the liquid reaches its boiling point. At this point, the molecules have so much energy that they can completely escape from each other as a gas. In the gaseous state, the molecules have enough kinetic energy to move about freely and independently, allowing the substance to expand to fill its container.
Understanding state changes on a molecular level helps explain why matter needs a continuous input of energy to transition between solid, liquid, and gas. The changes of state are driven by the kinetic energy gained by molecules through heating.
Kinetic Energy
Heating matter increases the kinetic energy of its molecules. Kinetic energy is the energy of motion. As heat is applied to matter, the molecules gain energy and start moving faster. The increased kinetic energy makes the molecules vibrate more intensely and move more rapidly.
On a microscopic level, heating causes the atoms and molecules to vibrate and move faster within the matter. The higher the temperature, the more kinetic energy the molecules have and the faster they vibrate and move around. This increased molecular motion accounts for many of the observable effects of heating matter, such as expansion, changing states, and chemical reactions.
Melting and Boiling Points
Melting and boiling points describe the temperatures at which phase changes between solid, liquid, and gas occur for a given substance. Specifically, the melting point is the temperature at which a solid begins to transform into a liquid, while the boiling point is the temperature at which a liquid turns into a gas.
The strength of intermolecular forces within the molecules of a substance determine its melting and boiling points. Stronger attractive forces between molecules require more energy to overcome, resulting in higher melting and boiling points. Substances like metals and ionic compounds with strong metallic and ionic bonds tend to have high melting and boiling points. Molecular compounds with weaker dipole-dipole interactions or London dispersion forces have lower melting and boiling points.
The pressure also affects melting and boiling points. Increased pressure raises the melting and boiling points of substances as more energy is required to create a phase change. Likewise, decreased pressure makes melting and boiling occur at lower temperatures. The purity of a substance influences its precise melting and boiling point as well. Impurities disrupt the substance’s intermolecular forces, causing variations in the expected melting and boiling point.
Chemical Changes
Heating can cause chemical changes and reactions as the energy added breaks molecular bonds. When bonds between atoms are broken, the molecules can rearrange and form new compounds. For example, heating sucrose (table sugar) to high temperatures causes it to decompose into carbon and water vapor through the breaking of bonds. The molecular formula changes from C12H22O11 for sucrose into CO2 and H2O.
The amount of energy required to break bonds is specific to each molecule and bond. Weaker bonds between atoms require less energy to break and will react at lower temperatures. Stronger molecular bonds require more energy input to break and reform into new compounds. This is why different substances undergo chemical changes at different temperatures as their molecular structures respond differently.
In chemical changes, the composition and properties are altered as the atomic structure and bonds change. This contrasts with physical changes where only the phase or shape is affected by heating. By understanding bond strengths in molecules, we can predict and control chemical changes by managing the energy input through heat.
Plasma State
When matter is exposed to extreme heating, such as temperatures exceeding thousands of degrees Celsius, it can transform into an ionized gas known as a plasma. This happens as the thermal energy causes the atoms in the gas to become so energetic that the electrons separate from the nuclei.
Plasma is often referred to as the fourth state of matter, distinct from solid, liquid and gas states. It has unique properties, displaying aspects of both gas and fluid states while also carrying an electrical charge and responding to electromagnetic forces.
Plasma does not have a definite shape or volume like solids and liquids. However, the ions and electrons in plasma are electrically charged particles that respond to electromagnetic fields. This enables plasma to be manipulated and controlled by magnetic and electric fields.
Plasma also conducts electricity well due to its free ions and electrons. It emits a color determined by the elements that make up the plasma. This effect is seen in neon signs, fluorescent lights and the aurora borealis.
Heat Transfer
There are three main ways heat transfers between objects:
Conduction
Conduction is the transfer of heat between objects that are in direct contact with each other. Heat energy is transferred between atoms and molecules through direct contact. Metals are good conductors of heat because their atoms can easily vibrate against each other and transfer energy.
Convection
Convection is the transfer of heat by the movement of fluids. As a fluid (liquid or gas) is heated, it expands, becomes less dense, and rises. Cooler, denser fluid then moves to take its place, creating a flow that transfers heat. Examples of convection include heating air currents and ocean currents.
Radiation
Radiation is the transfer of heat via electromagnetic waves. All objects emit infrared radiation based on their temperature. Hotter objects emit more radiation than cooler objects. Radiation can travel through empty space and does not require direct contact between objects for heat transfer.
Real World Examples
Heat is used in many real world applications to modify and utilize the properties of matter. Here are some examples:
Cooking – Applying heat is essential for cooking food. It can change the chemical structure of food, altering its flavor, texture, and nutritional profile. Cooking makes many foods safer and more digestible.
Heating Homes – Most home heating systems work by burning fuel to generate heat which is then transferred to living spaces. Common fuels used include natural gas, heating oil, propane, electricity, and wood.
Industrial Processes – Manufacturing industries use heat for applications like smelting metals, curing adhesives, drying painted surfaces, and molding plastics. Carefully managing heat is critical for achieving the desired material properties.
Controlling heat allows us to prepare food, stay warm, produce materials, generate electricity, and much more. Our mastery of heat underpins modern civilization and improves quality of life.
Conclusion
In summary, heat has a wide variety of effects on matter. The main effects discussed in this article include:
- Thermal expansion – heating matter causes expansion as the kinetic energy of atoms and molecules increases.
- State changes – heating or cooling matter can cause phase transitions between solid, liquid, gas and plasma states.
- Increased kinetic energy – heating increases the average kinetic energy and velocity of particles in matter.
- Melting and boiling points – specific temperatures where phase changes occur.
- Chemical changes – heat can initiate or accelerate chemical reactions and changes in matter.
- Plasma state – extremely high temperatures can ionize atoms into the plasma state.
- Heat transfer – heat moves from warmer objects to cooler ones through conduction, convection and radiation.
Understanding how heat affects matter helps explain many everyday phenomena and technical processes. The effects of heat are far-reaching across physics, chemistry, engineering and more. We have only scratched the surface in exploring such an important physical mechanism in this article.