What Is The Difference Between Radiant Heat And Light?
Radiant heat and light are types of electromagnetic radiation that are part of the electromagnetic spectrum. They are both wave-like forms of energy that travel through space or matter but differ in their wavelengths. Though they share some similar wave properties, radiant heat and light interact differently with materials, have distinct applications, and produce unique sensations.
This article will provide an overview of radiant heat and light, explaining the key differences between them. We will explore their wave characteristics, how they propagate, the ways they are emitted and absorbed, and how they create the sensations of warmth and vision.
Definitions
Radiant heat and light are both forms of electromagnetic radiation that travel in waves. However, they differ in their wavelength and frequency properties.
Radiant heat, also known as infrared radiation, is a type of electromagnetic radiation with longer wavelengths (between 700 nm to 1 mm) and lower frequencies than visible light. It is emitted by all objects above absolute zero temperature. Radiant heat is invisible to the human eye but can be felt as warmth on the skin.
Light, or visible light, is the visible spectrum of electromagnetic radiation that can be detected by the human eye. It has wavelengths between 380-700 nm. Visible light enables us to see color and brightness. Common sources include the sun, light bulbs, and computer/TV screens.
Definitions
Light is a form of electromagnetic radiation that is visible to the human eye. It includes all wavelengths of radiation that stimulate visual receptors in the retina, ranging from violet light with wavelengths around 380 nanometers to red light with wavelengths around 740 nanometers. Light allows us to see objects in the world around us. It travels in a straight line until it interacts with matter.
Radiant heat consists of infrared radiation, which are electromagnetic waves with longer wavelengths than visible light. Infrared radiation ranges from around 700 nanometers to 1 millimeter. We cannot see infrared rays, but we can detect them as heat. All objects emit some level of radiant heat based on their temperature.
Wave Properties
Radiant heat and light are both forms of electromagnetic radiation that travel as waves. They differ primarily in their wavelengths and frequencies. Wavelength refers to the distance between successive wave peaks. Frequency refers to how many cycles pass a point per unit of time.
Light waves have very short wavelengths, ranging from about 390 to 700 nanometers. Their frequencies are extremely high, around 430 to 750 trillion cycles per second. The visible wavelengths of light form what we see as the color spectrum.
Heat radiation has longer wavelengths than visible light, from about 700 nanometers up to 1 millimeter. Its frequencies range from 430 trillion down to 300 billion cycles per second. Infrared radiation, a type of heat radiation, has wavelengths just longer than those of visible red light.
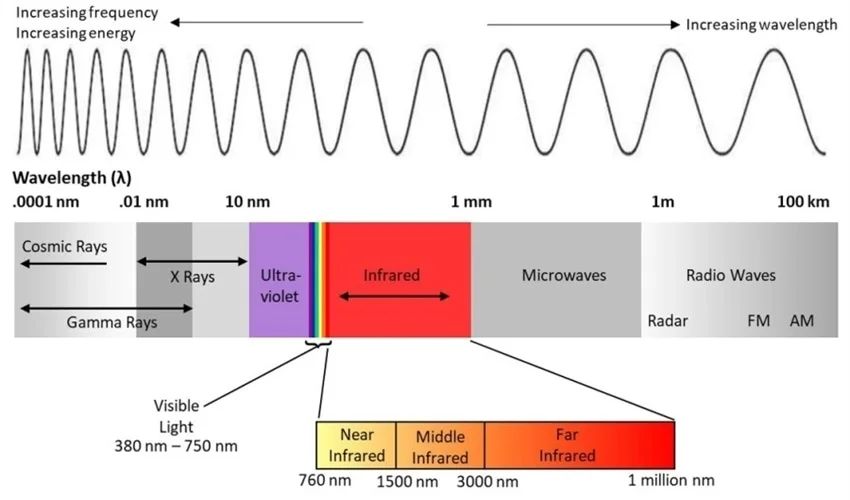
Wavelength and frequency are inversely related. Shorter wavelengths have higher frequencies, while longer wavelengths have lower frequencies. This relationship distinguishes heat radiation from light and underlies their different properties.
Propagation
Both heat and light travel in the form of waves. However, they differ in how they propagate through space:
Heat, or thermal radiation, propagates through electromagnetic waves in the infrared spectrum. It can travel through a vacuum as well as through air and other transparent media. Thermal radiation propagates outward in all directions from a heat source until it is absorbed by an object.
Light propagates as visible electromagnetic waves. It can only travel through transparent media like air, glass, or water. It cannot propagate through opaque objects or a vacuum. Light travels in straight lines from its source until it is reflected, refracted, or absorbed by an object.
So in summary, thermal radiation in the form of infrared waves can radiate through a vacuum while visible light cannot. Both types of waves propagate through transparent media in different ways until absorbed by an object.
Radiation
Both heat and light are forms of electromagnetic radiation that travel in waves. The main difference lies in their wavelengths. Heat radiation has longer infrared wavelengths, while visible light has shorter wavelengths. All objects emit infrared radiation related to their temperature. The hotter the object, the more energy it radiates at shorter wavelengths. This is why an incandescent light bulb emits both visible light and infrared heat. The sun emits light at a variety of wavelengths, including infrared wavelengths that we experience as heat. Greenhouse gases allow visible light to pass through but trap and absorb infrared radiation, causing the greenhouse effect that warms the Earth.
Sensation
Humans sense heat and light differently through specialized receptors. Heat is sensed through thermoreceptors in the skin that detect temperature changes. There are two main types of thermoreceptors – warmth receptors that detect temperatures above body temperature, and cold receptors that detect temperatures below body temperature. When these receptors detect a change in temperature, they send signals to the brain which perceives the change as hot or cold.
Light is sensed through photoreceptors called rods and cones in the retina of the eye. Rods work in low light conditions and allow us to see shapes and motion, while cones work in brighter light and allow us to see color. When light hits the photoreceptors, it causes a chemical reaction that sends signals to the visual cortex of the brain where the signals are processed into the images we see.
So while both heat and light are electromagnetic radiation, we perceive them through different mechanisms. Heat activates temperature receptors on the skin, while light activates photoreceptors in the eyes, allowing us to sense these types of energy in distinct ways.
Reflection
Light and radiant heat exhibit different reflective properties. Visible light tends to reflect off smooth, shiny surfaces like mirrors. Radiant heat, however, is often absorbed more readily into materials. For example, dark, dull surfaces will absorb radiant heat and light equally, but reflect very little visible light. On the other hand, shiny metal surfaces like aluminum foil will reflect visible light well but absorb much of the radiant heat that strikes it.
The different reflective properties of light and radiant heat come down to their wavelengths. Visible light waves have wavelengths of 400-700 nanometers. Radiant heat falls in the infrared region, with longer wavelengths from 700 nm to 1 mm. The longer infrared waves tend to pass through and be absorbed by materials rather than bouncing off smoothly like visible light. This distinction allows materials like glass to transmit visible light yet block radiant heat.
Understanding the reflective properties of light versus radiant heat allows us to tailor materials and surfaces to manipulate them differently. With the right coatings and textures, we can create surfaces that reflect visible light well for illumination while absorbing radiant heat to prevent excessive heating. These principles are applied in architecture and engineering to control heat and light within buildings and machines.
Absorption
The absorptive qualities of radiant heat and light differ substantially. Light is selectively absorbed by pigments in objects, giving them color. Objects that absorb all visible light frequencies appear black, while objects that reflect all visible light frequencies appear white. Radiant heat, on the other hand, is absorbed by all objects proportionally to their thermal conductivity. Good thermal conductors like metals will readily absorb radiant heat, quickly heating up when exposed to it. Poor thermal conductors like wood or plastic will absorb less and heat up more slowly. This difference in absorptive qualities means that light and radiant heat interact differently with various materials.
Applications
Radiant heat and light have many important practical applications that utilize their unique properties. Here are some key uses of radiant heat versus light:
Uses of Radiant Heat
Heating – Radiant heat is used for heating homes, buildings, and other spaces via radiators, baseboard heaters, and heating panels that transfer thermal radiation.
Cooking – Radiant heat is used for cooking food in ovens, toasters, broilers, and grills that apply thermal radiation to cook or toast food.
Industrial Processes – Radiant heat has industrial applications for heating, drying, curing, and treating materials with infrared lamps and burners.
Infrared Imaging – Radiant infrared heat emitted by objects is used for thermal imaging, night vision, and other imaging applications.
Uses of Light Radiation
Illumination – Visible light radiation is essential for lighting homes, workplaces, streets and enabling vision.
Displays – Light emission enables video displays, screens, TVs, monitors and other visual technologies.
Optical Communication – Fiber optic cables use light to transmit data for high-speed internet and telecommunications.
Solar Energy – Light radiation from the sun provides renewable solar energy through solar cells/panels and solar thermal systems.