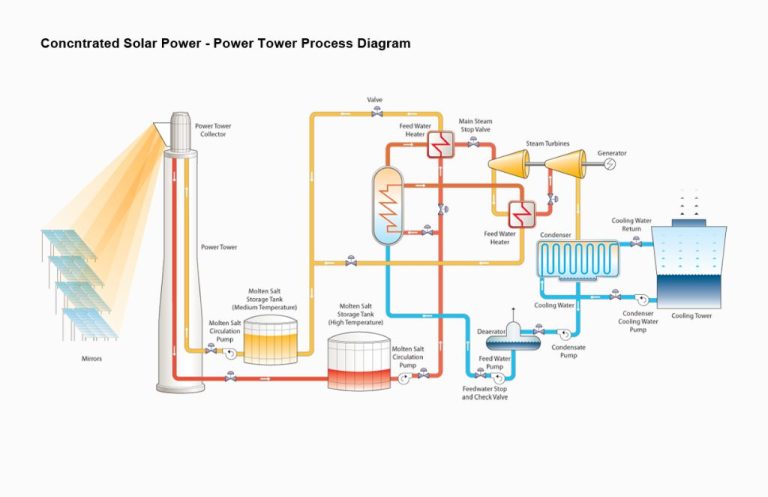
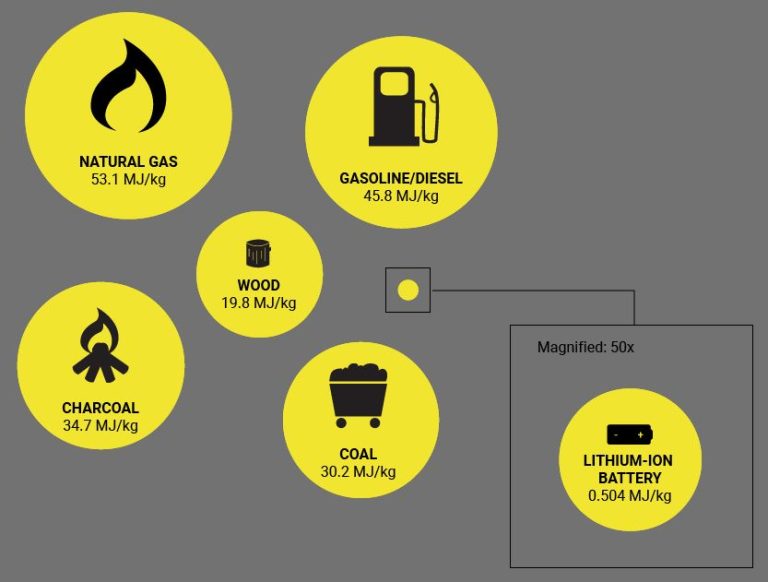
What Is The Definition And Example Of Thermal?
What is Thermal Energy?
Thermal energy refers to the total kinetic and potential energy of molecules within a substance. It is directly related to the temperature of matter and the movement of its particles. The higher the temperature of a material, the greater the thermal energy contained within it.
On an atomic level, all matter consists of atoms and molecules that are in constant motion. The kinetic energy of these particles produces thermal energy. Kinetic energy is energy associated with motion. The faster the particles move within a substance, the more kinetic energy they possess.
In addition to kinetic energy, atoms and molecules have potential energy stored within the bonds between them. Potential energy depends on the distance between interacting particles. As heat is applied to matter, the vibrations and spacing of the molecules increase, raising the potential energy.
Thermal energy is thus the sum of the kinetic and potential energy of all the microscopic particles in a material. This energy is directly tied to temperature, which is a measure of the average molecular kinetic energy. Materials with higher temperatures contain more thermal energy as their constituent atoms and molecules vibrate faster and have expanded atomic distances.
In summary, thermal energy arises from the motion and bonding of particles at the atomic scale. It manifests in observable ways through the temperature of matter.
Temperature vs Thermal Energy
Thermal energy, also known as heat energy, is the total kinetic and potential energy of atoms and molecules in a substance. It results from the motion of atoms and molecules in solids, liquids, and gases. Thermal energy can be transferred between substances through conduction, convection or radiation.
Temperature, on the other hand, is a measure of the average kinetic energy of atoms and molecules in a substance. It refers to how hot or cold a substance feels. Temperature is generally measured using a thermometer with standard units like Celsius, Fahrenheit, or Kelvin.
While related, thermal energy and temperature are distinct properties. Thermal energy refers to the total energy in a system whereas temperature indicates the average energy on a per particle basis. An object may contain a lot of thermal energy due to its mass, but have a low temperature if the energy is spread over many molecules.
For example, a bathtub of hot water has high thermal energy because it takes a lot of heat to raise the temperature of that much water. However, the water temperature may be low relative to other systems. On the other hand, the burner of a stove has a high temperature but lower total thermal energy than the bathtub of water.
Examples of Thermal Energy
Thermal energy refers to the internal energy present in matter as a result of the motion of molecules and atoms. It is often referred to as heat energy. There are different manifestations of thermal energy:
Heat – Heat is the transfer of thermal energy between substances of different temperatures. Heat flows spontaneously from a hotter substance to a colder substance. For example, when you boil water on a stove, the thermal energy from the stove heating element is transferred to the water, increasing the internal energy and temperature of the water.
Internal Energy – Internal energy is the total kinetic and potential energy of all the molecules within a substance. This energy is related to the random motions and vibrations of the atoms and molecules in the substance. The greater the internal energy, the more thermal energy is contained within the substance.
Thermal Radiation – Thermal radiation is the emission of electromagnetic waves from an object due to its internal energy. All objects with a temperature above absolute zero emit thermal radiation. An example is the heat felt from a fire – this is thermal radiation from the vibrating atoms and molecules within the flames being emitted outwards towards the surroundings.
Measuring Thermal Energy
Thermal energy is measured quantitatively using different units depending on the system. Some common units used to measure thermal energy include:
- Joules – The joule (J) is the SI unit of energy and work. It is defined as the energy required to produce one watt of power for one second. Joules are commonly used to measure thermal energy in scientific contexts.
- Calories – The calorie (cal) was originally defined as the amount of energy needed to raise the temperature of 1 gram of water by 1°C. Calories are commonly used to measure thermal energy in food.
- British Thermal Units (BTUs) – The BTU is a traditional unit of heat energy equal to about 1055 joules. It is commonly used to measure thermal energy content or energy consumption, especially in the United States.
Devices like calorimeters can precisely measure the thermal energy present in a system or transferred between systems. Understanding quantities of thermal energy is essential in many fields including engineering, physics, chemistry, nutrition science, and more.
Thermal Energy Transfer
Thermal energy can be transferred between objects or locations through three main mechanisms: conduction, convection, and radiation.
Conduction is the transfer of thermal energy between objects or substances that are in direct physical contact with each other. The better the conductor, the more rapidly heat will be transferred. Metals are good conductors while plastics and wood are poor conductors.
Convection is the transfer of thermal energy by the movement of matter. As an object is heated, the matter in that object moves, taking the thermal energy with it from one place to another. Convection occurs in liquids and gases.
Radiation is the transfer of thermal energy through electromagnetic waves or photons. All objects emit thermal radiation, with hotter objects emitting more than colder ones. Radiation does not require direct contact between the objects and can travel long distances through space.
Understanding how thermal energy transfers between objects and locations through conduction, convection, and radiation is key to applications like heating and cooling systems, weather forecasting, and more.
Thermal Energy in Matter
Thermal energy arises from the kinetic energy of atoms and molecules in matter. Atoms and molecules are constantly in motion, oscillating back and forth or vibrating. As they move, the atoms or molecules collide with one another. These collisions convert some of their kinetic energy into thermal energy.
The faster the atoms and molecules vibrate or move, the more thermal energy they possess. Thermal energy causes the atoms/molecules to move farther apart and spread out. This transfers energy throughout the material, increasing the internal energy of the system. The total kinetic energy of all the atoms or molecules in an object is related to the thermal energy and thus its temperature.
Materials differ in how their atomic/molecular structure allows thermal energy to spread. Metals tend to have free-flowing electrons that easily conduct thermal energy. Insulators have tightly bound atoms that resist thermal motion. Liquids and gases have atoms/molecules that can freely move and collide, diffusing thermal energy.
When thermal energy is added to matter, the atoms/molecules speed up. If thermal energy is removed, the atoms/molecules slow down. The more thermal energy in a material, the more active and energetic the atoms and molecules become.
Applications of Thermal Energy
Some of the most common applications of thermal energy in everyday life include:
Engines
Most engines, like those found in cars and airplanes, rely on thermal energy to operate. The burning of fuel creates heat which expands the gases and generates pressure that pushes the pistons in the engine. This thermal energy is then converted into mechanical energy that propels the vehicle.
Heating and Cooling
Thermal energy is harnessed in heating and cooling systems for buildings and refrigeration. Heat pumps and air conditioners use compression and expansion of gases along with the transfer of thermal energy to heat and cool interior spaces.
Cooking
Cooking relies on thermal energy to prepare food. Stovetops, ovens, and microwaves all generate heat that is transferred to the food, causing chemical and physical changes that make food safe and palatable to eat.
So in summary, thermal energy powers many essential technologies that keep us comfortable, help us travel, and allow us to enjoy delicious meals.
Thermal Energy Storage
Thermal energy storage (TES) refers to technologies that store thermal energy for later use. Two common methods of storing thermal energy are through batteries and phase change materials.
Thermal batteries store energy through a reversible thermochemical reaction. During the charging process, the chemical reactants absorb thermal energy and bonds between them are modified. The charging process can use a variety of heat sources including waste heat or solar thermal energy. When the battery is discharged, the reverse thermochemical reaction occurs and thermal energy is released. Thermal batteries allow storage of large amounts of energy over long durations.
Phase change materials (PCMs) store and release energy during the process of melting and freezing. As PCMs melt, they absorb heat without getting hotter. The phase change from solid to liquid allows large amounts of thermal energy to be stored. PCMs can store 5 to 14 times more heat per unit volume than conventional storage materials like water or rock. When the PCM solidifies again, it releases the stored latent heat. PCMs have the advantages of high storage density and the isothermal nature of the storage process.
Thermal energy storage allows excess thermal energy to be captured and used later when needed. It plays an important role in balancing energy supply and demand. TES can lead to improved efficiency and help integrate renewable energy sources into the grid.
Thermal Energy and Climate
Thermal energy plays an important role in Earth’s climate and weather patterns. The greenhouse effect is a natural process where greenhouse gases like carbon dioxide and methane absorb thermal radiation from the sun that has reached the Earth’s surface, causing the atmosphere to warm. This greenhouse effect helps regulate the Earth’s temperature and makes conditions livable for life on the planet.
However, human activities like burning fossil fuels have increased the levels of greenhouse gases in the atmosphere. This enhancement of the natural greenhouse effect leads to global warming, where more thermal energy is trapped rather than radiated back into space. According to NASA, global temperatures have increased by about 1.4°F since the late 19th century, with the most rapid warming occurring over the past 35 years.
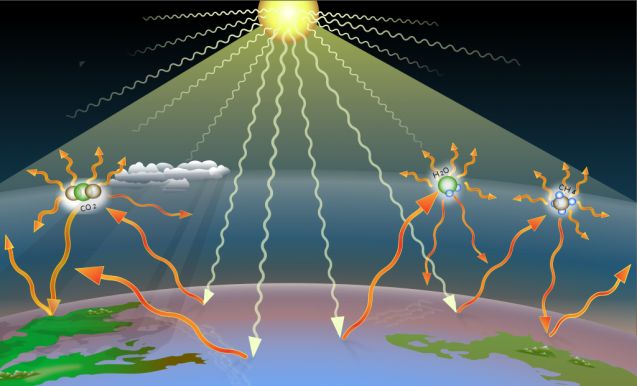
As global temperatures rise, climate change impacts become more pronounced. These include increased frequency and intensity of extreme weather events like heatwaves, hurricanes, floods and droughts. Melting polar ice caps and glaciers, rising sea levels, and changes in ecosystems are also consequences of excess thermal energy being trapped by greenhouse gases.
Mitigating climate change will require reducing greenhouse gas emissions and transitioning to clean, renewable energy sources that do not release excessive thermal energy into the atmosphere. Individuals can also help by minimizing their energy consumption and carbon footprint.
Fun Facts About Thermal Energy
Thermal energy has been crucial for humankind since the discovery of fire over a million years ago. Here are some fascinating facts about thermal energy through history:
The ancient Romans built the first geothermal heating system, with hot springs channeling thermal energy to heat homes and public baths. The ruins can still be seen at Aquae Sulis in modern-day England.
The first rudimentary steam engine was invented in the 1st century AD by the Greek engineer Heron of Alexandria. It was called the aeolipile and used steam to spin a sphere.
In the 17th century, German inventor Daniel Gabriel Fahrenheit created the first practical and reliable thermometer for measuring temperature. It led to the Fahrenheit temperature scale still used today.
The thermos flask, also known as a Dewar flask, was invented in 1892 by Scottish scientist Sir James Dewar. It allowed effective storage and transportation of thermal energy in beverages for the first time.
In 1911, Pyrex glass was invented by the Corning Glass Works company. The borosilicate glass had a very low coefficient of thermal expansion, allowing it to withstand sudden temperature changes without breaking.
The first microwave oven was invented in 1947 by American engineer Percy Spencer when he noticed a chocolate bar melting from accidental exposure to radar waves.
NASA used radioisotope thermal generators powered by decaying plutonium-238 to provide electricity aboard space probes too far from the Sun for solar power, beginning with the Transit-4A satellite in 1961.