What Is Energy Work And Heat?
What is Energy?
Energy is the ability to do work or produce heat. There are many different forms of energy including:
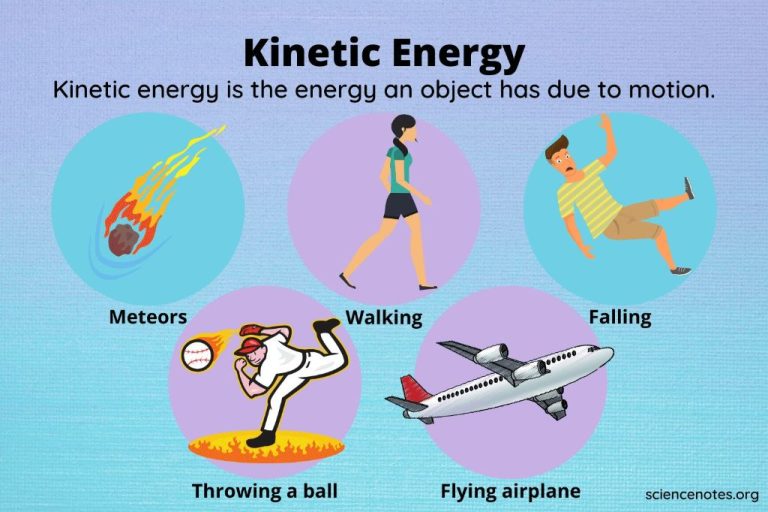
- Kinetic energy – the energy of motion
- Potential energy – stored energy based on an object’s position or arrangement
- Thermal energy – the internal energy of an object derived from the kinetic energy of its atoms and molecules
- Chemical energy – energy stored in the bonds between atoms and molecules
- Nuclear energy – energy stored in the nucleus of an atom
Other forms of energy include electromagnetic radiation, sound, elastic, and gravitational energy. Energy is generally quantified in units like joules, calories, electronvolts, etc. But at its core, energy is the capacity to do work or transfer heat.
What is Work?
Work is defined as force applied over a distance. When a force acts on an object and causes the object to move, work is being done. More specifically, work occurs when an object is moved in the same direction as the applied force.
For example, when you lift a heavy box from the ground, you apply an upward force on the box. As the box moves upward, work is done to raise it. The amount of work depends on the force exerted and the vertical distance the box is lifted.
Mathematically, work is calculated as:
Work = Force x Distance
Where Force is measured in Newtons (N) and Distance is measured in meters (m). The unit for work is the Joule (J), where 1 Joule = 1 Newton-meter.
In summary, work involves transferring energy from one object or system to another. When you lift the box, you transfer your muscular energy to increase the potential energy of the box. Without work being performed, energy would not be transferred between objects or systems.
What is Heat?
Heat is a form of energy that is transferred between objects or systems due to a temperature difference. When two objects at different temperatures come into contact, thermal energy flows spontaneously from the warmer object to the cooler one until they reach thermal equilibrium.
On a microscopic level, heat arises from the kinetic energy of atoms and molecules. As temperature increases, atoms and molecules vibrate and move faster, carrying more kinetic energy. This energy is transferred through collisions between rapidly moving particles. Heat always flows spontaneously from higher temperature to lower temperature objects. It does not require an external force or work to be done.
Heat is often confused with temperature, but they are distinct concepts. Temperature measures the average kinetic energy of molecular motion in a substance while heat refers to the total thermal energy present. Adding heat increases the temperature of a substance as it gains kinetic energy. Removing heat decreases the temperature as kinetic energy is lost.
Relationship Between Work and Heat
Work and heat are closely related concepts in physics. While they may seem distinct at first glance, work can actually generate heat under the right conditions. This relationship arises from the fact that work involves the transfer of energy, and heat is a form of energy transfer.
When a force displaces an object, work is done on that object. This transfers energy into the object. If the energy is then dissipated through friction or other resistive forces, it can be released from the object as heat. This is why rubbing your hands together generates warmth – the mechanical work done by the friction converts energy into heat. Machines also rely on this principle. As mechanical components rub against each other, the resistive work done converts some of the input energy into heat.
The reverse process is also possible. Heat transferred into an object can enable it to do work by harnessing the thermal energy. This is the operating principle behind heat engines. Fuel combustion generates heat which gets converted into useful mechanical work. So in essence, heat and work are interconvertible. Work can generate heat, while heat can enable work. They fundamentally represent different manifestations of energy transfer.
This relationship is formally expressed in the laws of thermodynamics. The First Law states that the change in internal energy of a closed system equals the heat supplied minus the work done by the system. So any work done on or by the system can affect the heat gained or lost. Understanding this link between work and heat is crucial across physics and engineering. It explains everything from engine efficiency to global warming. Work transfers energy, and heat represents the portion dissipated, so the two are intrinsically related.
Measuring Work and Energy
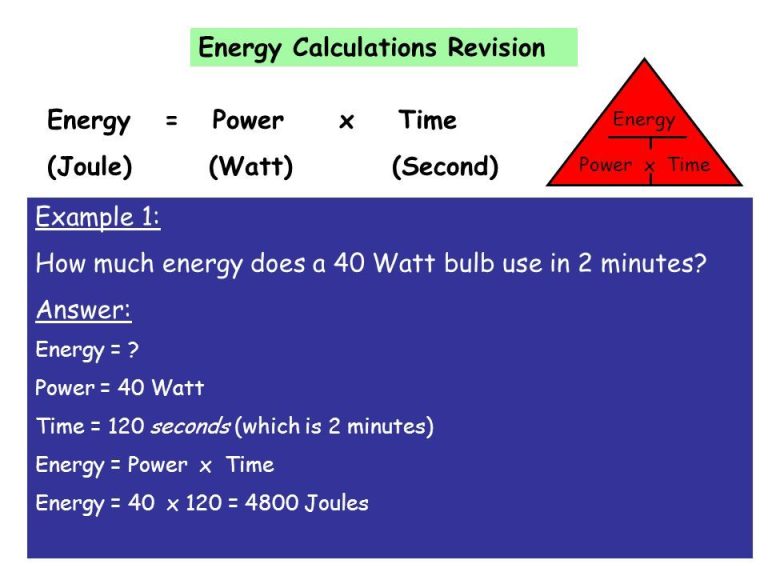
Work and energy are both measured using the same units. The standard unit is the joule (J).
One joule is defined as the work done by a force of one newton when it moves an object one meter in the direction of the force. For example, lifting a 1 kg object 10 meters against gravity does about 10 joules of work.
Energy is also measured in joules. Kinetic energy, potential energy, heat, and other forms of energy can all be quantified using joules.
Work and energy are connected by the work-energy theorem, which states that the net work (W) done on an object equals its change in kinetic energy (ΔK).
W = ΔK
This shows that work and energy are equivalent but in different forms – work is transferring energy into or out of a system.
Measuring Heat
Heat is measured using different units depending on the system. The most common units are calories or joules. A calorie (cal) is the amount of heat required to raise 1 gram of water 1 degree Celsius. A joule (J) is the SI unit of energy defined as the work required to produce one watt of power for one second.
Materials have different abilities to absorb heat. This is quantified by a property called specific heat capacity. Specific heat capacity measures how much heat must be absorbed or lost for a substance to change its temperature by 1 degree Celsius. Substances with higher specific heat capacities require more heat to change their temperatures. The specific heat capacity depends on the material and its phase (solid, liquid, or gas).
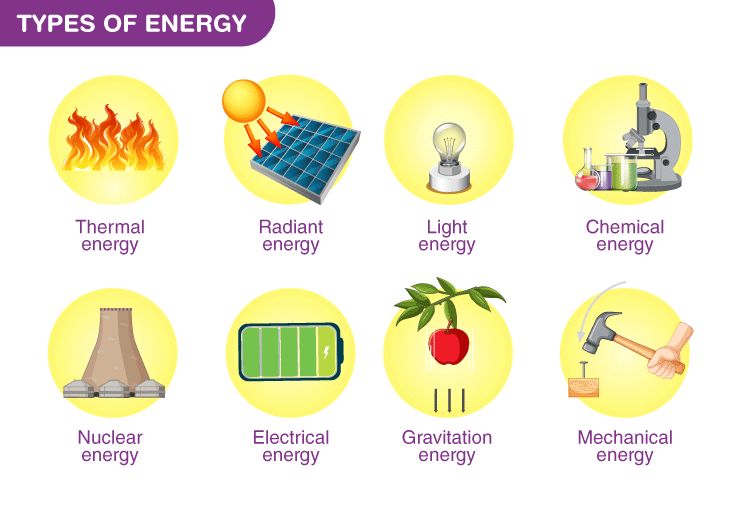
Forms of Energy
Energy exists in many different forms that can be grouped into categories. The main forms or categories of energy include:
Potential Energy
Potential energy is stored energy that depends on an object’s position or arrangement. Types of potential energy include gravitational potential energy from an object’s height, elastic potential energy stored in stretched or compressed springs, and chemical potential energy stored in the bonds between atoms and molecules.
Kinetic Energy
Kinetic energy is energy of motion. Objects have kinetic energy if they are moving. The faster or heavier an object is, the more kinetic energy it has. Common examples include the kinetic energy of a ball or other moving object.
Thermal Energy
Thermal energy, also called heat, is the internal energy of an object or system resulting from the motion of atoms and molecules. Thermal energy flows from warmer objects to cooler ones until equilibrium is reached. All matter contains some thermal energy, even at temperatures close to absolute zero.
Chemical Energy
Chemical energy is stored in the bonds between atoms and molecules. Chemical reactions release energy when new bonds are formed or absorb energy when bonds break. This energy can often be harnessed and used. Common examples of stored chemical energy include fossil fuels, batteries, and food.
Nuclear Energy
Nuclear energy comes from tiny particles inside the nuclei of atoms. Nuclear reactions, such as fission and fusion, can release vast amounts of nuclear energy. Examples include nuclear power plants, which use nuclear fission, and the sun, which generates energy through nuclear fusion.
Electrical Energy
Electrical energy results from the movement of charged particles, like electrons. Examples include static electricity and the current flowing through outlets, wires, and electrical devices. Electrical energy can easily be converted into other types of energy and transported over long distances.
First Law of Thermodynamics
The First Law of Thermodynamics is one of the fundamental laws of physics. It states that energy cannot be created or destroyed, but can only be transformed from one form into another.
This law explains the relationship between heat and work. When a process occurs in a thermodynamic system, it involves the transfer of energy either through heat or work. The First Law states that the total energy of the system remains constant, even as energy transforms between heat and work. For example, when mechanical work is done on a system, some of the energy is transferred as heat. The total energy remains the same, even as the heat and work portions change.
The First Law of Thermodynamics is essential for understanding the flows and transformations of energy in scientific and engineering systems. It sets an absolute limit on the efficiency of heat engines and other thermodynamic processes. This fundamental law applies universally across all disciplines involving thermodynamic processes and energy transfer.
Examples of Work and Heat
Work and heat are important concepts that have many everyday applications.
One of the most common examples of work is the internal combustion engine in cars and other vehicles. The exploding fuel inside the cylinders does work by pushing the pistons. This work gets transferred to the wheels to move the car forward. The faster the explosion and the bigger the pistons, the more work gets done.
Another everyday example is the work done by friction. When two surfaces rub against each other, the friction does work by converting kinetic energy into heat. This is why rubbing your hands together warms them up – the friction is doing work.
Boiling water is a great example of heat transfer at the molecular level. As the water is heated, the added energy gets transferred to the water molecules as kinetic energy, making them vibrate and move faster. When the water reaches its boiling point, the molecules have enough energy to turn to vapor and escape from the liquid as steam.
In all of these examples, work and heat are transferring energy and making things happen at both the macro and microscopic levels. Understanding these concepts helps explain many common phenomena around us.
Importance and Applications
Thermodynamics plays a fundamental role in physics and engineering. The laws of thermodynamics have wide ranging practical applications in science and technology.
Some of the key areas where thermodynamics concepts like work, heat and energy are applied include:
- Power generation – Thermodynamics principles enable the design and operation of power plants that generate electricity from heat sources like coal, natural gas or nuclear fuel.
- Heating and cooling – HVAC (heating, ventilation and air conditioning) systems rely on thermodynamics to provide heating or cooling efficiently.
- Transportation – Internal combustion engines in automobiles, aircraft and other vehicles convert heat into mechanical work based on thermodynamic cycles.
Thermodynamics also has important implications in fields like chemistry, aerospace engineering, mechanical engineering and electrical engineering. The laws of thermodynamics put constraints and limits on what is possible in a wide range of technologies and processes.
Overall, thermodynamics concepts like work, heat and energy flow form the backbone of many engineering systems and technologies that power modern civilization.