What Happens During An Energy Transfer?
Energy transfer refers to the process by which energy is passed from one object, system, or place, to another. It involves transforming energy from one form to another or transferring energy between objects through various mechanisms.
Energy transfers occur constantly all around us. For example, chemical energy in food is transferred into kinetic energy as animals move, thermal energy is transferred from the Sun to the Earth to provide warmth, and energy stored in batteries is transferred into electricity to power devices. Understanding the principles of energy transfer allows us to harness it for useful work.
This article will provide an overview of the different forms energy can take, the various mechanisms by which it can be transferred between objects or systems, the law of conservation of energy which governs the process, as well as real-world examples and applications of energy transfers.
Forms of Energy
Energy comes in many different forms that can be categorized into two main types: potential energy and kinetic energy. Potential energy is stored energy that has the potential to do work, while kinetic energy is energy in motion. The different forms of energy include:
- Potential Energy
- Chemical – Energy stored in the bonds between atoms and molecules. Examples are batteries, biomass, petroleum, natural gas, and food.
- Nuclear – Energy stored in the nucleus of an atom. Nuclear power plants split atoms to release this energy.
- Gravitational – Objects high up have potential energy that can be released when they fall.
- Elastic – Energy stored when an object is compressed or stretched. Examples are stretched springs and rubber bands.
- Kinetic Energy
- Radiant – Electromagnetic energy including light, radio waves, microwaves, X-rays, and gamma rays. Light from the sun is an example.
- Thermal – The internal energy of an object related to the random motion of its atoms and molecules. Heat is an example.
- Motion – The energy an object possesses by being in motion. Examples are wind, sound, moving water, and moving vehicles.
Energy Transformation
Energy transformation refers to the process by which energy changes from one form to another. For example, a car engine transforms the chemical energy stored in gasoline into mechanical energy that powers the car. Some common energy transformations include:
– Chemical energy to thermal energy – Burning wood in a fireplace transforms the chemical energy stored in the wood’s molecular bonds into thermal energy (heat) and light energy.
– Solar energy to electrical energy – Solar panels convert sunlight into electricity.
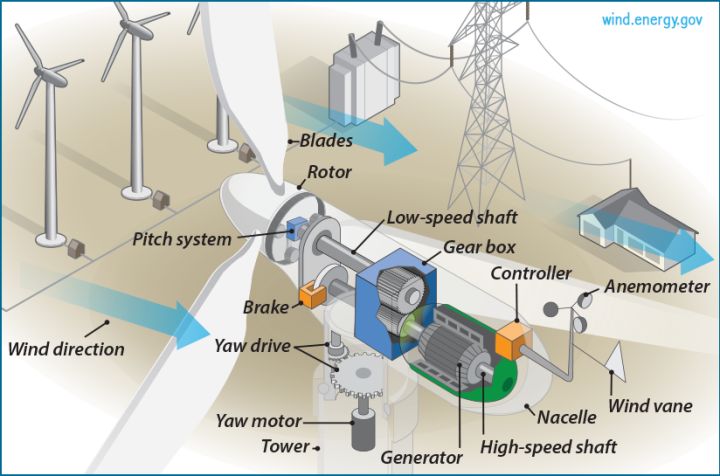
– Mechanical energy to electrical energy – Wind turbines and hydroelectric generators use mechanical energy from wind and falling water to produce electricity.
– Electrical energy to light energy – Light bulbs transform electrical energy into visible light and heat.
– Chemical energy to mechanical energy – Batteries use chemical reactions to produce electricity to power devices.
– Nuclear energy to thermal energy – Nuclear power plants split uranium atoms to release tremendous amounts of heat which is used to boil water into steam that drives turbines.
Energy transformation enables useful applications of different energy forms. For example, without chemical to electrical energy transformation it would not be possible to power modern society. Understanding energy transformations is key to designing efficient energy systems.
Transfer Mechanisms
There are three main mechanisms by which energy can be transferred from one place or system to another: heat, work, and radiation.
Heat is the transfer of thermal energy between objects or systems that are at different temperatures. Heat flows spontaneously from higher temperature objects to lower temperature objects until thermal equilibrium is reached. Examples of heat transfer include touching a hot stove, radiant heat from the sun warming the earth, and heat conduction through the walls of a house.
Work is a mechanism of energy transfer that involves a force moving an object over a distance. Mechanical work transfers energy into or out of a system when an external force is applied. For example, walking up stairs, hitting a tennis ball, and generating electricity all require work to be done.
Radiation is the emission and transmission of energy through electromagnetic waves or photons. Radiant energy can be transferred across empty space and does not require a transport medium like air or water. Common examples include visible light from the sun, radio waves, microwaves, and x-rays. Radiation allows the sun’s thermal energy to reach the Earth.
Understanding how heat, work, and radiation transfer energy is key to explaining phenomena from heat engines to photosynthesis. The mechanisms provide pathways for energy to move between objects, locations, and systems in our universe.
Law of Conservation of Energy
The law of conservation of energy states that energy can be neither created nor destroyed, only transformed or transferred from one form to another. This means the total energy in an isolated system always remains constant.
For example, when a ball drops, its potential energy is transformed into kinetic energy. The chemical energy contained in wood changes into thermal energy and light energy when burned. In both cases, the total amount of energy before and after the transfer remains the same.
This law holds true for all isolated systems with no external energy inputs or outputs. However, most real world systems are open systems that exchange energy with their surroundings. In these cases, the total system energy may change, but the law still applies to the isolated system consisting of the original system plus surroundings.
The law of conservation of energy is one of the foundational laws of physics and helps explain phenomena from chemical reactions to biological processes. It shows that energy cannot disappear – it only changes form. This law allows tracking energy transfers systematically and quantifying them mathematically in fields like thermodynamics and engineering.
Real World Examples
Energy transfers occur constantly in our everyday lives. Here are some common examples:
Photosynthesis – Plants convert light energy from the sun into chemical energy that is stored in glucose molecules. This energy is later released when animals eat plants, or when plants respire.
Internal combustion engines – The chemical energy stored in fuel is converted into thermal energy through combustion, which is then transformed into mechanical energy that powers the engine.
Electricity generation – Energy sources like coal, natural gas, nuclear, wind, solar etc. are used to turn turbines connected to generators. This converts mechanical energy into electrical energy.
Metabolism – The chemical energy stored in food is broken down through metabolic processes into thermal and mechanical energy that allows our bodies to function.
Lighting – Electrical energy is converted into light energy and thermal energy by lighting fixtures like bulbs, tubes, and LEDs.
In all these examples, energy is transferred from one form into another. The law of conservation of energy mandates that no energy is created or destroyed in the process.
Energy Transfer Efficiency
When energy is transferred from one object or system to another, some of the useful energy tends to become dissipated or lost as unusable forms of energy. Often this occurs due to friction, resistance, sound, or heat that is produced but not doing useful work. The efficiency of an energy transfer refers to the amount of useful energy that gets transferred versus the total amount of energy expended in the process.
For example, when chemical energy stored in gasoline gets transformed into kinetic energy that propels a car forward, some of that energy is lost as heat and sound from the engine and drivetrain. Only around 20-30% of the potential energy in gasoline ends up actually moving the car down the road. The rest is wasted energy that does not contribute to the car’s motion.
When electricity travels through power lines and gets transformed into light energy from a bulb, around 10% is lost as heat dissipated in the wires and bulb. The efficiency is the useful light energy output divided by the total electrical energy input.
Understanding where energy gets lost or wasted is important for designing more efficient systems that maximize useful energy transfer and minimize energy dissipation. Even small gains in efficiency can lead to major energy savings when scaled up across entire industries and societies. There are always thermodynamic limits and tradeoffs, but improving efficiency is a key strategy for reducing energy consumption and costs.
Impacts and Applications
Energy transfers have wide-ranging impacts on technology and everyday life. Understanding energy transfers enables engineers to design more efficient systems like engines, power plants, batteries, and solar panels. The applications of energy transfers are seen all around us.
For example, internal combustion engines in cars and generators convert the chemical potential energy in fuel into kinetic energy to move pistons and mechanical energy to turn the wheels or generator. Power plants use heat from burning coal or nuclear fission to boil water, transforming thermal energy into mechanical energy via steam turbines that drive electrical generators. Batteries and fuel cells utilize electrochemical reactions to produce an electric current that powers electronic devices.
Knowledge of energy transfers also allows us to tap into renewable sources like the sun, wind, tides, and geothermal heat and convert them into useful electricity. Solar panels turn light energy into electrical current through the photovoltaic effect. Wind turbines capture the kinetic energy of moving air and transform it into rotational mechanical energy to drive a generator. Similar principles are applied to harvest energy from waves, rivers, and the Earth’s internal heat.
Understanding energy transfers is key to designing more efficient systems that reduce wasted energy, lower costs, and lessen environmental impacts. Advances like electric vehicles, high-efficiency appliances, and smart grids rely on optimizing energy transfers at every stage. As technology progresses, insights into energy transfers will help us make the most of our energy resources.
Common Misconceptions
Despite our reliance on energy transfers in everyday life, there are a few common misconceptions about how they work:
Energy is Used Up During Transfers
Many people believe that energy is “used up” or disappears when transferred from one object to another. This is inaccurate – the law of conservation of energy states that energy can neither be created nor destroyed, only transformed from one form to another. While some energy escapes or is dispersed as heat during transfers, the total amount of energy remains constant.
Friction Eliminates Kinetic Energy
It’s often thought that kinetic energy from motion is simply lost or eliminated due to friction. In reality, the kinetic energy is transformed into thermal energy in the form of heat. The movement energy isn’t destroyed but rather changes form. This heat created by friction is why surfaces often feel warm after rubbing together.
Chemical Energy is Fundamentally Different
While there are many forms of energy, they all follow the same basic rules of transfer and transformation. Chemical energy may seem more complex, but it too obeys conservation of energy – chemical energy released in reactions changes into other forms like heat, light, and motion. Recognizing that all energies behave similarly deepens understanding of energy transfers.
Summary
To recap, an energy transfer refers to the process of energy changing from one form to another. There are many different forms of energy such as mechanical, thermal, chemical, nuclear, electrical, radiant, and more. The law of conservation of energy states that energy cannot be created or destroyed – it can only change forms. There are several mechanisms by which energy transfers occur, including conduction, convection, radiation, and more. Real world examples of energy transfers include chemical energy in food transforming into thermal energy and kinetic energy during digestion or electrical energy converting into light and thermal energy in a lightbulb. The efficiency of energy transfers can vary greatly depending on the system and process. Understanding energy transfers is very important, as they are fundamental to many applications and technologies in fields like engineering, chemistry, physics, and more.
In summary, energy transfers describe the processes of energy changing between different forms, which occurs through various mechanisms. The total energy remains constant, in accordance with the law of conservation of energy. Studying energy transfers provides key insights into our natural world and helps enable many useful technologies and applications.