What Do You Mean By Transformation Of Energy?
Energy transformation refers to the process of changing energy from one form to another. Energy can take many different forms, such as kinetic energy, potential energy, thermal energy, electrical energy, chemical energy, nuclear energy, and radiant energy. When energy is transformed from one type to another, the total amount of energy remains the same. Energy transformation happens constantly around us.
Some examples of energy transformations include a ball gaining kinetic energy as it falls and converts gravitational potential energy into motion (kinetic energy), electrical energy being converted into light and heat energy by a light bulb, chemical energy in gasoline being converted into thermal energy and kinetic energy as an engine burns fuel, and radiant light energy from the sun being converted into chemical energy through photosynthesis.
Energy transformation is important because it allows us to transfer energy into usable forms. For example, chemical energy in a battery is transformed into electrical energy to power devices, thermal energy produced by burning fuel converts water into steam to spin turbines for electricity generation, and light energy from the sun is converted and stored as chemical energy by plants.
Understanding energy transformation allows us to harness energy sources and convert them into useful types of energy that power our modern world.
Potential and Kinetic Energy
Potential energy is energy that is stored in an object due to its position or arrangement. For example, a book sitting on a table has potential energy due to gravity acting on its mass. The higher the position of the object, the greater its potential energy. Kinetic energy is the energy an object has due to its motion. The faster the object moves, the greater its kinetic energy.
Potential and kinetic energy are linked concepts that can transform into each other. For example, when you hold a ball up high, it has gravitational potential energy. When you drop the ball, its potential energy transforms into kinetic energy as it accelerates towards the ground. When the ball hits the ground and bounces back up, the kinetic energy transforms back into gravitational potential energy. This back and forth transformation happens continuously until energy escapes the system through friction, sound, deformation, or other effects.
Other examples of potential to kinetic energy transformations include a compressed spring releasing its energy and a pendulum swinging back and forth. The transformation between potential and kinetic energy applies to many mechanical systems and is an important concept in physics.
Chemical to Thermal Energy
Chemical energy is the potential energy stored in the bonds between atoms and molecules. It is energy stored within a substance that can be released during a chemical reaction. Common examples of stored chemical energy are in the food we eat, wood, gasoline, batteries and other fuels.
Thermal energy, also called heat, is the energy associated with the random motions of atoms and molecules. It arises from the kinetic energy of atoms and molecules in a substance due to their random motions and vibrations. As the motions and vibrations increase, more thermal energy is present in a substance.
The transformation of chemical energy to thermal energy frequently occurs during combustion, or burning. Combustion is a high-energy chemical reaction in which a fuel reacts with an oxidant, such as oxygen, to release energy in the form of heat and light. The stored chemical energy in the fuel is converted into thermal energy during this exothermic reaction. For example, when wood burns, the chemical energy stored in the wood’s organic molecules is released as thermal energy. The thermal energy produced can be used for heating and cooking. Combustion is an important energy transformation that allows us to utilize the stored chemical energy in substances like wood, coal, oil and natural gas.
Mechanical to Electrical Energy
Mechanical energy is the energy associated with the motion and position of an object. For example, a ball at the top of a ramp has potential mechanical energy due to its position. As the ball rolls down the ramp, the potential mechanical energy gets converted into kinetic energy associated with the motion of the ball.
Electrical energy is energy derived from electric charges or flow of electrons. It involves the movement of electrons from one atom to another within substances like wires and motors.
Mechanical energy can be converted into electrical energy through electromagnetic induction. This is done by generators which rely on the principle of electromagnetic induction. In electromagnetic induction, motion between a magnetic field and a conductor induces a flow of electrons or electricity within the conductor.
For example, in a wind turbine the rotational mechanical energy of the turbine spins a shaft connected to magnets within a generator. As the magnets spin past copper wire coils, it causes electrons in the copper to flow and generate electricity. The mechanical rotation gets transformed into electrical energy.
Similarly, in a hydroelectric dam, the mechanical energy of falling water pushes the blades of a turbine which then spins a shaft connected to a generator to produce electricity.
The reverse can also occur where electrical energy is converted into mechanical energy such as in an electric motor. Overall, generators allow the transformation of movement and mechanical energy into usable electrical energy.
Radiant to Thermal Energy
Radiant energy is the energy carried by electromagnetic waves. Examples of radiant energy include visible light, ultraviolet light, infrared radiation, radio waves, and gamma rays. On Earth, the most common source of radiant energy is the Sun. The Sun radiates an enormous amount of electromagnetic energy in all directions.
When radiant energy from the Sun reaches Earth, it interacts with matter on our planet and transforms into other forms of energy. One of the most important energy transformations is the conversion of radiant energy into thermal energy. Thermal energy is the internal energy of matter that arises from the motion of atoms and molecules. Heating matter results in an increase in its thermal energy.
As radiant energy from the Sun strikes objects on Earth, the electromagnetic waves interact with the atoms and molecules, causing them to vibrate faster. This increase in molecular motion corresponds to a rise in temperature. In this way, radiant electromagnetic energy is converted into thermal energy and heat. This transformation of radiant solar energy into heat is essential for warming Earth’s land, air, and water. It provides the thermal energy that drives weather, ocean currents, and the water cycle. Radiant to thermal energy conversion allows life to thrive on Earth.
Nuclear to Thermal Energy
Nuclear energy is the energy stored in the nucleus of an atom. It is released when atomic nuclei split apart in a process called nuclear fission or when smaller nuclei fuse together in nuclear fusion. Nuclear power plants use nuclear fission to produce electricity.
In a nuclear power plant, a reactor contains uranium fuel rods that undergo controlled nuclear fission. This fission process heats water to extremely high temperatures, creating steam. The high-pressure steam then spins a turbine, which turns a generator to produce electricity. So nuclear power plants transform nuclear potential energy into thermal energy in the form of steam, which is then used to generate electricity.
The process begins when uranium atoms are bombarded with neutrons, causing their nuclei to split apart into lighter elements. This nuclear fission releases kinetic energy in the form of heat and neutrons that go on to cause more fission reactions. The heat is used to boil water, producing high-pressure steam that spins a turbine. So nuclear energy is transformed into thermal energy before being converted into electrical energy.
Chemical to Radiant Energy
Chemical energy stored in molecules can be converted into radiant light and heat energy through combustion reactions. For example, when wood burns in a campfire, the chemical energy stored in the wood’s molecules is released. This energy is emitted in the form of light and heat.
Specifically, the chemical bonds between atoms in the molecules break apart. This releases energy that was stored in the bonds. The combustion reaction between the wood and oxygen in the air converts the chemical potential energy into kinetic energy in the form of heat and light. The heat causes the wood and air around it to increase in temperature. The light is visible in the flames. This light is a form of radiant energy created by the excited electrons in the atoms and molecules as they return to lower energy states after the bonds break.
The color of the light depends on the materials burning. For example, a campfire generates an orange/yellow glow as the soot particles and chemical byproducts of wood combustion emit particular wavelengths of light. The radiant energy spreads outward rapidly in the form of electromagnetic waves that travel at the speed of light. This allows the heat and light to spread quickly throughout the surrounding environment.
In summary, chemical energy stored in the molecular bonds of a material like wood can be converted into radiant light and heat energy through combustion reactions. The visible light and infrared radiation carry energy away from the fire through electromagnetic waves. This energy transfer is seen clearly during the burning process of a campfire or other fire.
Gravitational Potential to Kinetic Energy
One of the most common examples of energy transformation that we experience in everyday life is the conversion of gravitational potential energy to kinetic energy. Gravitational potential energy is the stored energy an object possesses due to its vertical position in a gravitational field. For instance, a book sitting on a high shelf has more gravitational potential energy than when it is resting on a table. This stored energy has the potential to be converted into kinetic energy, the energy of motion.
When an object falls, its gravitational potential energy gets converted into kinetic energy. As gravity pulls the object down, it accelerates and gains increasing speed. This increase in velocity is actually the gain in kinetic energy. A simple example is dropping a ball from a rooftop. At the start when the ball is held steady, it has gravitational potential energy due to its height above the ground. As soon as it is dropped, this potential energy gets transformed into kinetic energy as the ball accelerates under gravity. The kinetic energy continues increasing as the ball picks up speed until it hits the ground.
The gain in kinetic energy comes directly from the loss of gravitational potential energy. This energy transformation follows the law of conservation of energy, which states that the total energy in a closed system remains constant. The gravitational potential energy gets converted but no energy gets created or lost in the process. Understanding such energy transformations allows engineers to design structures and machines that utilize gravitational forces effectively.
Photosynthesis – Light to Chemical Energy
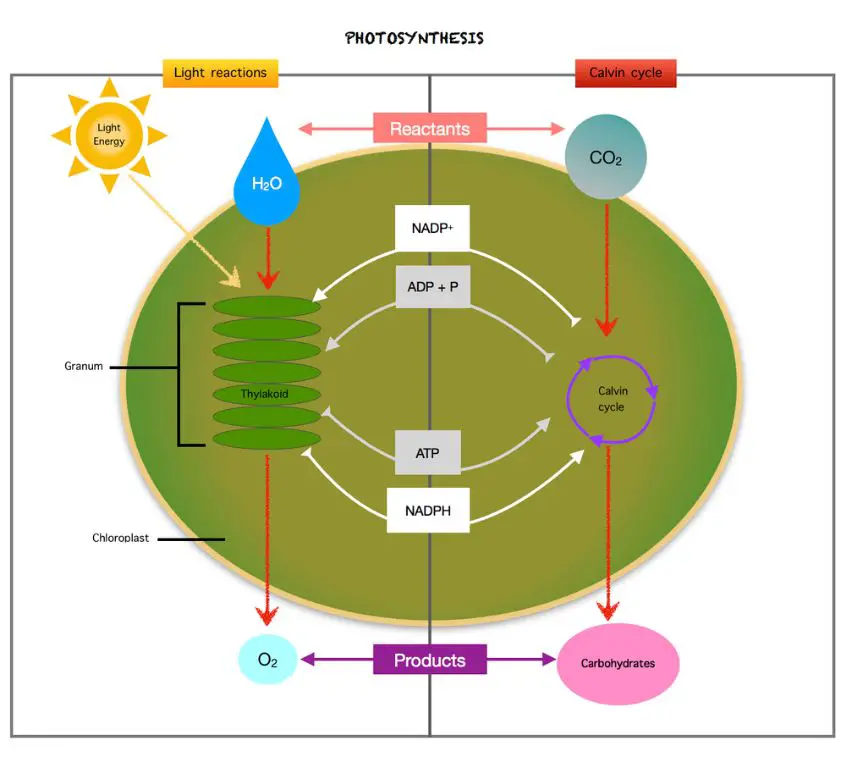
Photosynthesis is the process by which plants use sunlight, carbon dioxide, and water to create their own food and energy. This process converts light energy into chemical energy that the plant can use as fuel.
During photosynthesis, plants capture photons of light from the sun using chlorophyll in their leaves and other green parts of the plant. The chlorophyll absorbs the light energy, which initiates a series of chemical reactions.
In the first stage of photosynthesis, light energy is converted into chemical energy and ATP, which stores the energy. The plant uses this energy along with carbon dioxide from the air to produce glucose, a simple sugar. The glucose molecule contains far more chemical energy than the carbon dioxide and water that were used to produce it.
The glucose can then be broken down by the plant to release energy for growth and other cellular processes. Any excess glucose is often stored as starch or converted into other forms of carbohydrates. Overall, the light energy from the sun is transformed into chemical energy in the form of sugars and other organic molecules through the wondrous process of photosynthesis.
Conclusion
In this piece, we explored several important examples of energy transformations that occur regularly in our world. We looked at how stored potential energy can turn into kinetic energy, such as when an object falls due to gravity. We saw how chemical energy in fuel and food can turn into thermal energy and motion. Mechanical energy can be transformed into electrical energy, which powers much of our modern society. Radiant energy from the sun transforms into thermal energy when it heats objects. Nuclear reactions in power plants convert nuclear energy into useful thermal energy.
Photosynthesis in plants is a particularly essential energy transformation, turning light energy from the sun into chemical energy stored in glucose molecules. This stored chemical energy is then consumed by organisms up the food chain. We also saw how chemical energy in batteries can be converted into radiant energy in light bulbs.
Energy transformations power our human world every single day, from the cars we drive to the lights that illuminate our homes. As our technologies advance, we discover new and improved ways to transform energy from one form to another. By better understanding these fundamental energy transformations, we gain insights into how to more efficiently harness different energy sources for human needs.