What Converts Heat Energy To Light Energy?
Heat energy and light energy are two different forms of energy. Heat energy is the energy transferred between objects or systems due to their temperature difference. It flows from a hotter body to a colder one until they reach thermal equilibrium. Light energy, on the other hand, is the energy carried by photons, which are particles representing a quantum of light. Light is a form of electromagnetic radiation that is visible to the human eye.
In this article, we will discuss various processes that can convert heat energy into light energy. When heat energy is provided to certain materials or systems, the added thermal energy can excite their electrons to higher energy states. As the electrons drop back down to their ground states, photons are emitted in the form of visible light. We will explore several examples of such light-emitting processes, ranging from heated metal filaments to chemical reactions to living organisms.
Incandescence
Incandescent light bulbs produce light through a process called incandescence. Inside an incandescent bulb is a thin wire filament, usually made of tungsten. When electricity passes through the filament, it heats up the filament to extremely high temperatures. At these high temperatures, the tungsten filament starts to glow and give off light. This glowing effect from heat is called incandescence. The hotter the filament gets, the brighter it glows.
In an incandescent bulb, the tungsten filament has a high resistance to electrical current. This resistance causes the filament to heat up significantly as electricity flows through it. The glass bulb surrounding the filament contains either a vacuum or an inert gas, which prevents the hot tungsten from oxidizing and burning up. The glass also allows the visible light emitted by the glowing filament to pass through. In this way, incandescent bulbs convert the heat from the resistive tungsten filament into visible light that illuminates the surrounding area. This simple but clever use of electrical resistance and heat has powered electric lighting for over a century.
Fluorescence
Fluorescent bulbs utilize mercury vapor and phosphor coating to produce light through fluorescence. Inside the glass tube of a fluorescent bulb, there is a small amount of mercury vapor and an inert gas. When electricity flows through the tube, it excites the mercury atoms, causing them to emit ultraviolet light.
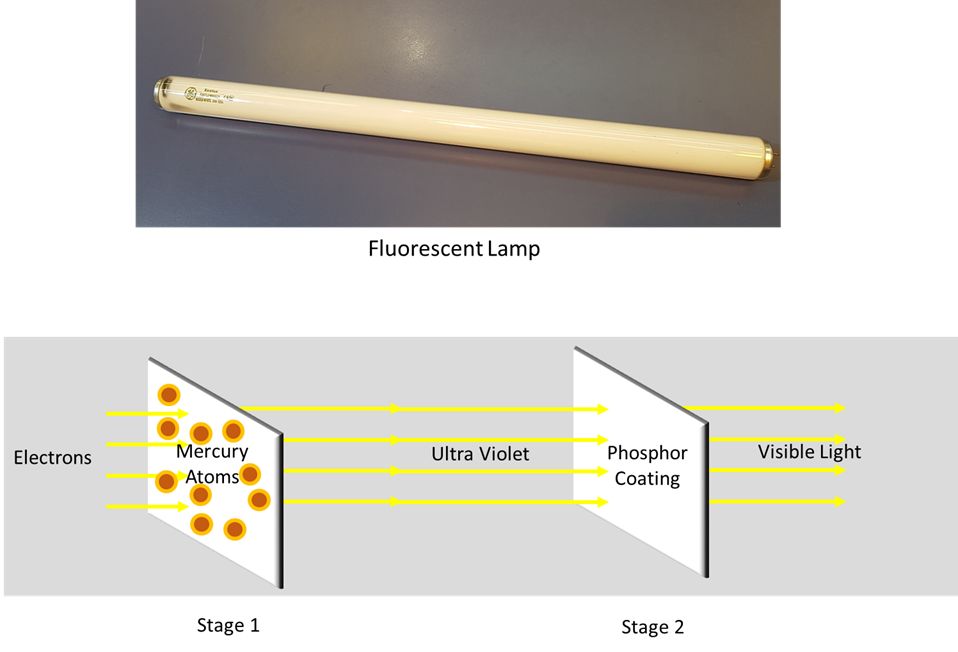
The inside of the tube is coated with a phosphor powder which absorbs the ultraviolet light and re-emits it at longer wavelengths that are visible to the human eye. Different phosphor compounds emit different colors of visible light. The interaction of the mercury vapor and phosphor coating is what causes the bulb to produce visible light through fluorescence.
Compared to incandescent bulbs that rely on hot filaments producing light through incandescence, fluorescent bulbs are much more energy efficient. They require less electricity to excite the mercury vapor and produce UV light than is needed to heat a filament to extreme temperatures. This makes fluorescent lighting a good choice when energy efficiency and cost savings are a priority.
Chemiluminescence
Chemiluminescence is light emission that results from a chemical reaction. A common example of chemiluminescence that many people have experience with are glow sticks. Glow sticks contain two separate compartments within a flexible plastic tube – one compartment contains the chemical dye such as luminol or cyalume, and the other contains the activator chemical such as hydrogen peroxide. When the tube is bent to mix the contents of the two compartments, the resulting chemical reaction produces energy in the form of light rather than heat. The glowing effect can last from several minutes up to a few hours depending on the intensity of the reaction. Other examples of chemiluminescence include fireflies, which produce light from the reaction of luciferase and luciferin within their bodies. This process allows fireflies to communicate through their blinking light patterns during mating rituals. In the laboratory, chemiluminescence is also widely used for medical diagnostics and analysis.
Bioluminescence
Bioluminescence is the production and emission of light by a living organism. It is a form of chemiluminescence where a luciferin substrate is oxidized by the enzyme luciferase in the presence of molecular oxygen, generating light. Bioluminescence is common among marine vertebrates and invertebrates, as well as some species of fungi, microorganisms, and terrestrial arthropods such as fireflies.
Some examples of bioluminescent organisms include:
- Fireflies – Fireflies produce light through a chemical reaction that occurs in specialised light-emitting organs called lanterns. The enzyme luciferase acts on the luciferin substrate in the presence of ATP and oxygen to produce oxyluciferin in an electronically excited state, which emits light when it returns to the ground state.
- Glow worms – Glow worms are actually a type of fly larva that generate light through a similar bioluminescent reaction as fireflies, involving the luciferase enzyme and luciferin substrate.
- Deep sea fish – Many fish that inhabit the dark ocean depths produce their own light through symbiotic bioluminescent bacteria. These include anglerfish, lanternfish, flashlight fish, and dragonfish.
- Dinoflagellates – These tiny marine plankton display bioluminescence when disturbed. Movement stimulates bioluminescent chemicals within vacuoles to mix and emit light.
- Mushrooms – Some fungi like the honey mushroom can emit a faint glow through bioluminescent compounds and reactions.
So in summary, bioluminescence allows certain organisms to produce biological light through internal chemical reactions involving luciferase enzymes and luciferin substrates. This light can serve purposes like attracting prey, defense, camouflage, or communication in the dark environments these creatures inhabit.
Candoluminescence
Candoluminescence refers to light emission that occurs when certain materials, such as inorganic salts and minerals, are heated. Specifically, candoluminescence produces light when crystals are heated to high temperatures, typically between 500-1000°C.
The light emission happens because heating the crystal provides energy that excites electrons in the crystal lattice into higher energy states. When the electrons fall back down to lower energy states, they emit photons in the form of visible light. The color of the emitted light depends on the specific electronic transitions taking place in the crystal structure.
Common materials that exhibit candoluminescence include fluorite, apatite, and calcite crystals, as well as white-hot lava and molten inorganic salts. The effect can be observed by strongly heating these materials, either with a flame or other high temperature heat source. The light produced is often quite bright and can persist as long as the material remains hot.
Candoluminescence has been utilized by humans to create interesting light displays and effects. For example, synthetic crystals and salt mixtures have been developed that glow specific colors when heated, allowing for customized pyrotechnic displays. The phenomenon also has uses in geology, allowing researchers to identify the mineral content of lava rocks based on their visible light emission.
Crystalloluminescence
Crystalloluminescence is a phenomenon where light is emitted from certain crystals when they are deformed. This occurs when the crystalline structure of the material is changed through mechanical stress like crushing, cleaving, or scratching. The process causes the excitation of electrons within the crystal lattice, and when these electrons return to their ground state, photons in the visible light spectrum are emitted.
Some materials that exhibit crystalloluminescence include sucrose, quartz, and zinc sulfide. The color of light emission depends on the specific composition and structure of the crystal. For example, when white sucrose crystals are crushed, a faint blue glow can be observed. This is likely due to molecular cleavage that generates unstable radical ions which then recombine and emit light. Similarly, fracturing quartz releases photons in the ultraviolet range.
The crystalloluminescent effect has applications in testing mechanical stress distribution, acting as an indicator of crystal damage. The light emission can help visualize how and where a crystal sample fractures under mechanical loading. The phenomenon also has uses in physics research, helping study electron excitations and bonding within crystal lattices. Overall, crystalloluminescence provides insights into the atomic and molecular interactions that occur as crystals undergo deformation and structural changes.
Triboluminescence
Triboluminescence is the production of light through the breaking of chemical bonds when mechanical stress is applied. Some examples of triboluminescent materials include wintergreen, sugar, and adhesive tape. When a wintergreen candy is crushed or fractured, the stress on the crystallized structure causes electrons to be freed and emit photons in the visible light spectrum. This results in a brief flash of blue light. The effect can be seen by crushing wintergreen Life Savers candies or Wint-O-Green mints in a dark room.
Adhesive tapes made of acrylic or cellophane produce blue light when unrolled, ripped, or peeled apart forcefully. This is due to the polymers breaking down and releasing energy in the form of light. Similarly, when a pile of granulated sugar is ground in a mortar and pestle, a faint light can be seen emitting from the crushing grains. Mechanical deformation of the sugar crystals releases energy stored in the crystal lattice, giving off a momentary glow.
Thermoluminescence
Thermoluminescence is a phenomenon where certain crystalline materials emit light when heated. This effect occurs in materials like quartz, calcium fluoride, and sodium chloride that contain defects in their crystalline structure. These defects form when the material is exposed to radiation, which displaces electrons into higher energy states within the crystal lattice. The displaced electrons are trapped and held in place by the defects.
When the thermoluminescent material is heated, the trapped electrons gain enough thermal energy to escape from the defects and return to lower energy states. As they transition back down, the electrons emit photons in the form of visible light. The amount of light emitted can indicate the total dose of radiation absorbed by the material over time. This makes thermoluminescence useful for radiation dosimetry and dating archaeological artifacts like pottery.
The intensity of thermoluminescence depends on the type and concentration of defects in the irradiated material. More defects and trapped electrons will produce brighter luminescence when heated. The color of the emitted light also varies based on the specific electronic transitions occurring in the material. By carefully heating thermoluminescent samples and analyzing the light, scientists can study the crystal defects and the material’s prior radiation exposure.
Conclusion
There are several ways that heat energy can be converted into light energy. Some of the main ways include incandescence, where heat applied to a solid causes it to glow, like heating up a metal wire until it emits light. Fluorescence involves absorbing high energy radiation like UV light and re-emitting it as lower energy visible light, like how fluorescent tubes work. Chemiluminescence relies on chemical reactions to produce energy in the form of light. Bioluminescence is light emission from living organisms through chemical reactions. Other methods involve applying mechanical energy like friction or pressure to emit light, known as triboluminescence and mechanoluminescence. Phase transitions like crystallization can also release energy as light in some materials. Overall, the conversion of heat into light relies on supplying enough energy to raise electrons into excited states so that when they fall back down, photons are emitted.