What Causes An Energy Change In Matter?
Energy is the capacity to do work. Energy can change forms within matter, but it cannot be created or destroyed. There are two main types of energy associated with matter: potential energy and kinetic energy.
Potential energy is stored energy based on matter’s position or composition. For example, a ball at the top of a hill has potential energy due to gravity. Kinetic energy is energy associated with motion. For instance, a rolling ball has kinetic energy.
There are several ways that energy can change forms in matter, including through endothermic reactions, exothermic reactions, phase changes, chemical reactions, and nuclear reactions. The laws of thermodynamics govern these energy transformations.
This article will provide an overview of the different causes of energy changes in matter, including potential and kinetic energy, endothermic and exothermic processes, phase changes, chemical reactions, nuclear reactions, and the laws of thermodynamics.
Potential Energy
Potential energy is the stored energy an object has due to its position or chemical structure. There are several types of potential energy:
Gravitational Potential Energy
Objects have gravitational potential energy when raised to a height due to the force of gravity acting on their mass. A book on a high shelf has more gravitational potential energy than a book on the floor. As the book falls, gravitational potential energy is converted to kinetic energy.
Elastic Potential Energy
Elastic potential energy is stored in materials that can be deformed through stretching or compression, like rubber bands. When stretched, the atoms are pulled apart from their equilibrium positions, storing energy.
Chemical Potential Energy
Chemical potential energy is energy stored in the bonds between atoms in molecules and compounds. Foods like sugars and fats contain chemical potential energy that is released when the molecules are broken down during digestion.
Kinetic Energy
Kinetic energy is the energy of motion. An object that has motion – whether it is vertical or horizontal motion – has kinetic energy. Kinetic energy increases as the object’s speed increases. Some examples of kinetic energy include:
- The motion of a bicycle
- The motion of a baseball being thrown
- The vibration of molecules (heat)
- The movement of sound waves
In each of these examples, the motion or vibration of the object represents kinetic energy. The faster the motion, the higher the kinetic energy. For example, a bicycle moving at 10 mph has more kinetic energy than a bicycle moving at 5 mph. Increasing an object’s speed increases its kinetic energy.
Endothermic Reactions
Endothermic reactions are chemical reactions that absorb heat energy from the surroundings. These reactions require an input of energy to proceed, so they cause the temperature of the surroundings to decrease. Some examples of endothermic reactions include:
Dissolving ammonium nitrate in water
When ammonium nitrate dissolves in water, the process absorbs heat. The dissolution breaks bonds between ammonium nitrate molecules, which requires an input of energy. As a result, the temperature of the solution decreases.
Photosynthesis in plants
Photosynthesis involves using sunlight energy to convert carbon dioxide and water into glucose and oxygen. This process stores energy in the glucose molecules, so it requires an input of heat and causes a decrease in the surrounding temperature.
Melting a solid into a liquid
The phase change process of melting requires energy input to break the structured bonds in a solid. Melting ice into liquid water absorbs heat from the environment, causing the surrounding temperature to drop.
In summary, endothermic reactions draw in heat from their surroundings as they proceed. Common examples include dissolving ammonium nitrate, photosynthesis, and melting solids into liquids.
Exothermic Reactions
Exothermic reactions are chemical reactions that release energy in the form of heat or light. These reactions give off more energy than they take in, resulting in a net release of energy. Some examples of exothermic reactions include:
- Combustion or burning – This includes burning fossil fuels like coal, oil, and natural gas to produce energy. The combustion of gasoline in a car engine is also exothermic.
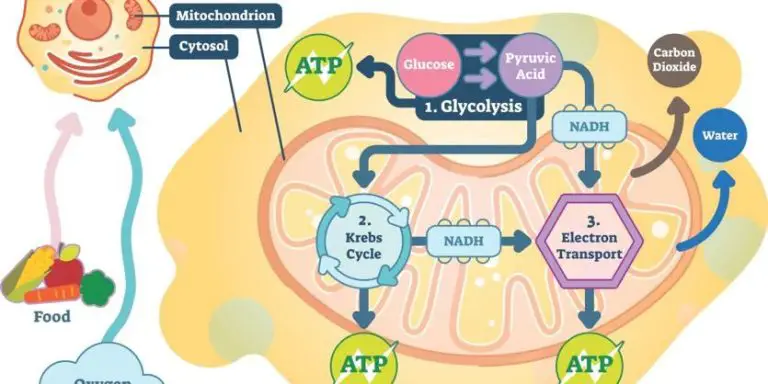
- Digestion of food – The metabolic breakdown of food like carbohydrates, fats, and proteins releases energy that is used by the body. Cellular respiration is an exothermic process.
- Many oxidation reactions like rusting of iron and the tarnishing of silver.
- Acid-base neutralization – When an acid and base react, salt and water are formed which gives off heat.
In exothermic reactions, energy is released because the chemical bonds in the products contain less energy than the bonds in the reactants. Exothermic reactions play an important role in chemical and biological processes that involve the transfer of energy.
Phase Changes
A phase change is a physical change from one state of matter to another without changing its chemical composition. Some examples of phase changes are:
- Melting – solid to liquid
- Freezing – liquid to solid
- Evaporation – liquid to gas
- Condensation – gas to liquid
- Sublimation – solid to gas
- Deposition – gas to solid
These phase changes occur because energy, usually in the form of heat, is added or removed from the substance. For example, when a solid melts into a liquid, its molecules gain enough energy to break out of the fixed positions they had in the solid state and are able to move around freely. The reverse occurs during freezing, as molecules lose energy and become locked into solid state positions. Phase changes commonly occur during heating and cooling processes.
Chemical Reactions
Chemical reactions involve the rearrangement of atoms as reactants are converted into products. This reorganization and forming/breaking of chemical bonds leads to energy changes. Exothermic reactions release energy, usually in the form of heat, light, or sound. For example, combustion reactions like burning fuels involve oxygen reacting with hydrocarbons to produce carbon dioxide and water and give off heat. On the other hand, endothermic reactions absorb energy. Electrolysis uses electricity to split water molecules into hydrogen and oxygen gas. In both exothermic and endothermic reactions, the total energy of the universe remains constant. Energy is neither created nor destroyed, but converted from one form to another.
Nuclear Reactions
Nuclear reactions involve changes in the nuclei of atoms. There are three main types of nuclear reactions that can cause energy changes in matter:
Nuclear Fission
Nuclear fission is the splitting of a large atomic nucleus into smaller nuclei. This reaction releases an enormous amount of energy, as some of the mass of the original nucleus is converted into energy according to Einstein’s equation E=mc2. Nuclear fission reactions power nuclear reactors and atomic bombs.
Nuclear Fusion
Nuclear fusion is the joining of two light atomic nuclei to form a heavier nucleus. This reaction requires a tremendous input of energy to overcome the repulsive forces between the nuclei. If achieved, nuclear fusion releases even more energy than fission. The Sun produces energy through fusion of hydrogen nuclei into helium.
Radioactive Decay
Radioactive decay is the spontaneous breakdown of an unstable atomic nucleus into a more stable form. This happens by the emission of high-energy particles such as alpha particles, beta particles, or gamma rays. Radioactive decay releases energy as the mass of the decaying nucleus is converted into energy.
In summary, nuclear reactions involve changes in atomic nuclei that can release enormous amounts of energy, according to Einstein’s famous equation that relates energy and mass. Nuclear reactions are some of the most powerful energy changes found in nature.
Laws of Thermodynamics
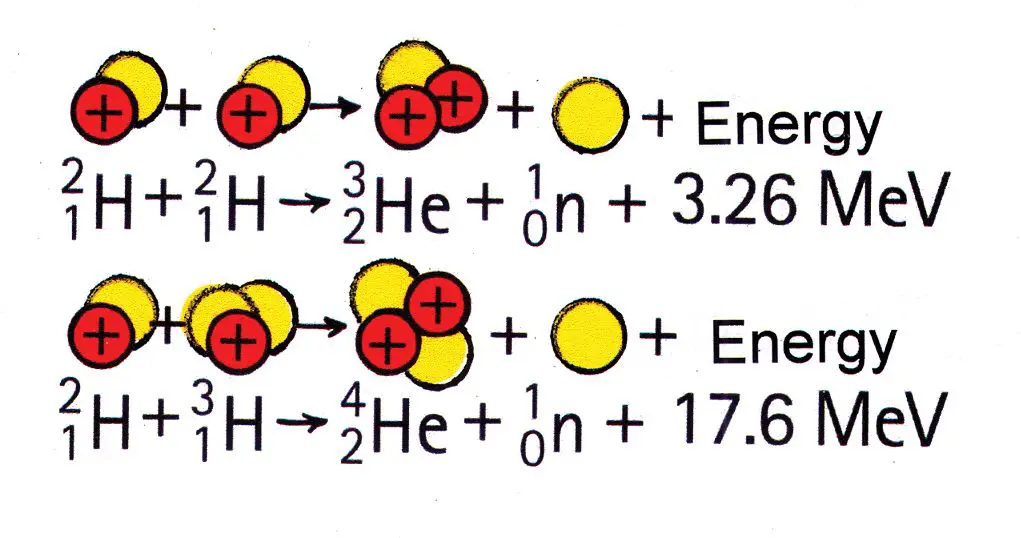
The laws of thermodynamics describe the relationships between thermal energy, mechanical work, and heat transfer. The laws help explain how and why energy changes take place in matter. There are three major laws of thermodynamics:
The First Law of Thermodynamics states that energy cannot be created or destroyed; it can only be transformed from one form to another. This is also known as the law of conservation of energy. For example, when a bowl of hot soup cools down, the thermal energy is not destroyed – it is transferred to the surroundings.
The Second Law of Thermodynamics states that in any closed system, the total entropy can never decrease over time. Entropy is a measure of the disorder or randomness of a system. An increase in entropy means the energy in a system is spread out uniformly. Because usable energy requires differences in temperature, pressure, etc., increased entropy leads to reduced availability of usable energy.
The Third Law of Thermodynamics states that a system’s entropy approaches a constant value as its temperature approaches absolute zero. Absolute zero is the theoretical lowest limit on the temperature scale, at which point a system has no thermal energy.
Understanding these fundamental laws helps explain phenomena like why hot things cool down over time, why some chemical reactions occur spontaneously while others do not, and why perpetual motion machines are impossible. The laws place real boundaries around what forms of energy transfers are possible.
Conclusion
In summary, there are various ways that energy can change in matter. The main categories are:
- Potential Energy – Energy stored in an object due to its position or shape
- Kinetic Energy – Energy of motion
- Endothermic Reactions – Reactions that absorb heat
- Exothermic Reactions – Reactions that release heat
- Phase Changes – Energy absorbed or released when matter changes state
- Chemical Reactions – Energy absorbed or released when chemical bonds are formed or broken
- Nuclear Reactions – Energy absorbed or released when atomic nuclei are changed
These various energy changes are governed by the Laws of Thermodynamics. The First Law states that energy is conserved, it cannot be created or destroyed. The Second Law states that the entropy of the universe always increases. Understanding the different ways energy can change in matter provides insight into the natural world.