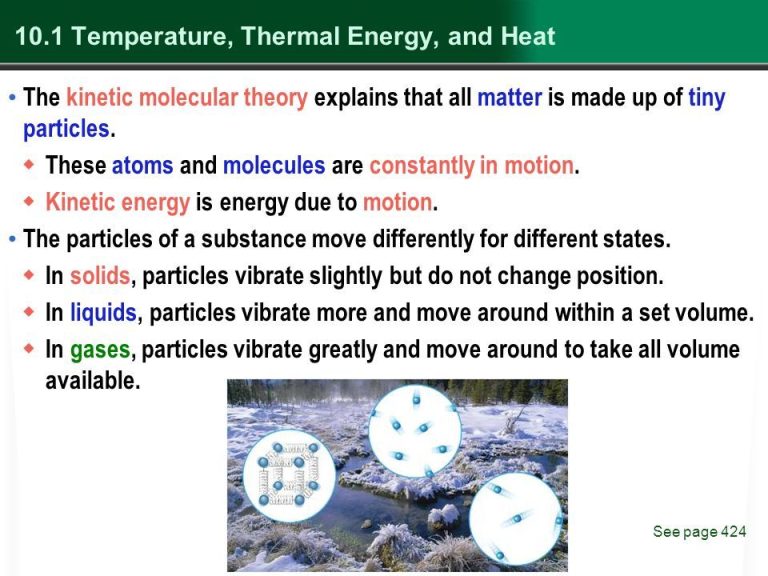
Is Thermal An Example Of A Form Of Energy?
What is Thermal Energy?
Thermal energy refers to the total kinetic energy and potential energy of molecules in a substance. It involves both heat and temperature. Thermal energy is present in all matter and can be transferred between objects through processes like conduction, convection, and radiation.
We encounter examples of thermal energy in our everyday lives. The warmth from sunshine, the heat from a campfire flame, the cooling sensation of an ice cube, and the coziness of a heated blanket are all examples of thermal energy. Thermal energy flows spontaneously from warmer places to colder places. Understanding thermal energy allows us to harness it for useful applications like power generation, heating and cooling systems, and more.
Forms of Energy
Energy comes in many different forms that can be categorized into two main types – potential energy and kinetic energy. Potential energy is stored energy that has the potential to do work. Some examples of potential energy include chemical energy stored in batteries, gravitational energy stored in water held behind a dam, or elastic energy stored in a stretched rubber band.
Kinetic energy is energy of motion. Moving objects contain kinetic energy, such as a rolling ball, flowing water, or wind. When objects that contain potential energy are released, the potential energy changes into kinetic energy. For example, when a dam opens, the potential energy stored in the water behind the dam is released and changed into kinetic energy as the water flows out rapidly.
Other important forms of energy include:
- Mechanical energy – the sum of an object’s kinetic and potential energy
- Electrical energy – energy from electric charges or flow of electrons
- Chemical energy – energy stored in the bonds between atoms and molecules
- Radiant energy – energy in the form of electromagnetic waves, like light
- Nuclear energy – energy stored in the nucleus of atoms
- Thermal energy – the internal energy of substances from the motion of atoms and molecules
Energy is constantly being converted from one form into another. For example, chemical energy in gasoline is converted into mechanical energy to move a car. The car’s mechanical energy is then partially converted into thermal energy due to friction. This energy conversion is how work gets done in our universe. Understanding these different forms of energy and how they change is key to innovating and harnessing energy efficiently.
Why Thermal Energy is a Form of Energy
Thermal energy is considered a form of energy because it possesses the ability to do work and cause change, just like other forms of energy such as mechanical, electrical, chemical, nuclear, and more. Thermal energy refers specifically to the kinetic energy of molecules within a substance or system.
Thermal energy is directly tied to the temperature of matter. As molecules vibrate faster and move more rapidly within a substance, the thermal energy increases, resulting in a rise in temperature. So temperature serves as a measurement and indicator of the thermal energy that is present and contained within the molecular motions of a system.
Because thermal energy comes from the kinetic activity of molecules, it can be transferred between substances and converted into other forms of energy. For example, the warmth from a hot stove element can cause water in a pot to boil, converting the water’s thermal energy into kinetic energy as it turns into steam. This demonstrates the ability of thermal energy to do work and instigate changes, confirming its categorization as a bonafide form of energy.
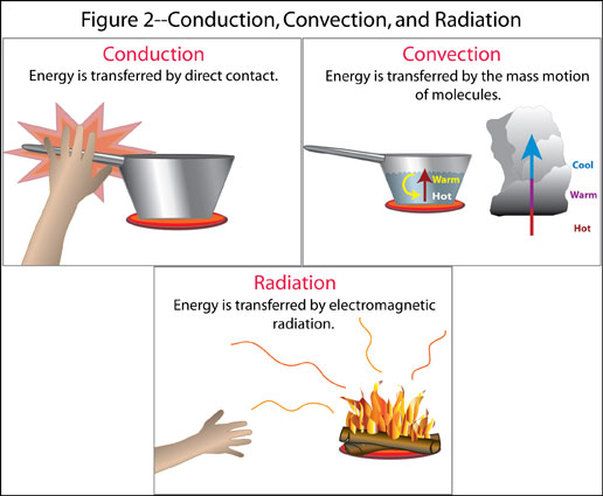
Thermal Energy Transfer
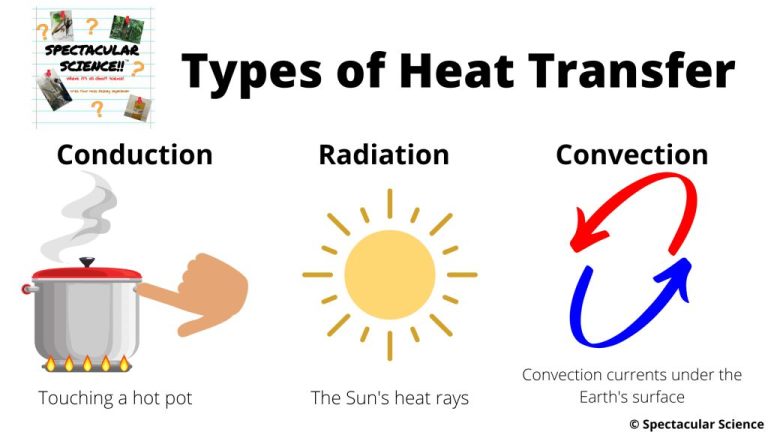
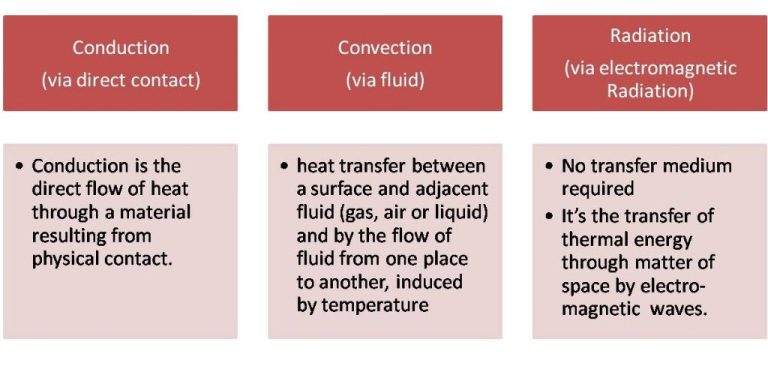
Thermal energy is transferred in three main ways – conduction, convection, and radiation.
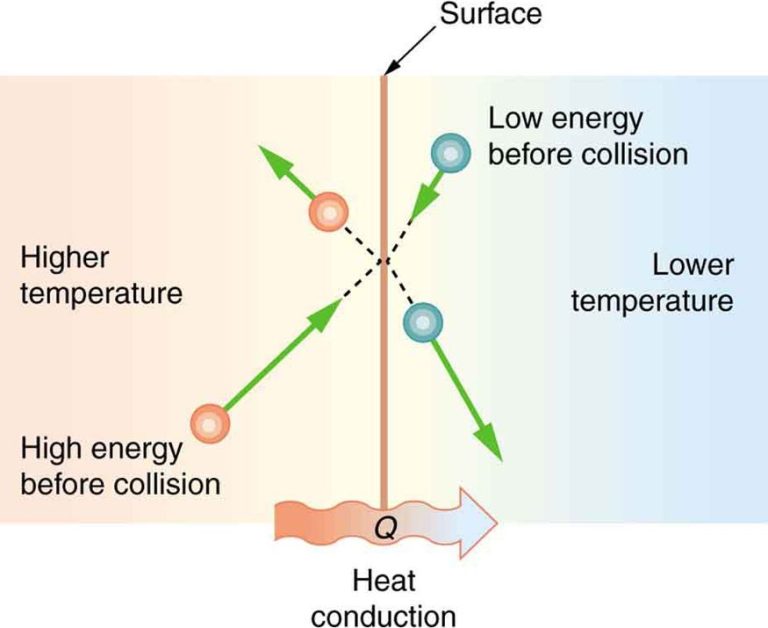
Conduction is the transfer of thermal energy through direct contact between materials. Metals are good conductors of thermal energy. An example is a spoon heating up when placed in hot soup. The thermal energy transfers from the hot soup to the cooler metal spoon through conduction.
Convection is the transfer of thermal energy via movement of fluids. As a fluid is heated, it expands, becomes less dense, and rises. Cooler, denser fluid then moves to take its place. This sets up a circulation pattern that transfers heat. Examples include heating and airflow from radiators, stoves, and fires.
Radiation is the transfer of thermal energy through electromagnetic waves. No direct contact is needed. The sun transfers thermal energy to the Earth through radiation. A hot burner element on an electric stove transfers thermal energy to a pan through radiation.
Real-world examples of thermal energy transfer include heating homes and buildings with furnaces, heating water with boilers and hot water heaters, and cooking food on stoves. Engines also rely on thermal energy and convert it to mechanical energy.
Using Thermal Energy
Thermal energy has many practical applications in our everyday lives. Some of the most common uses of thermal energy include:
Heating and Cooling: Heating and air conditioning systems rely on thermal energy to raise or lower air and water temperatures in homes, offices, and other buildings. Furnaces, boilers, heat pumps all use thermal energy to provide heating, while air conditioners and refrigerators remove thermal energy for cooling.
Cooking: Appliances like ovens, stovetops, and microwaves all use thermal energy to heat up and cook food. The heat transforms the chemistry of food by denaturing proteins, caramelizing sugars, and more – resulting in delicious meals!
Engines: Most engines, like internal combustion engines in cars and gas turbines in jet engines, generate thermal energy from burning fuel. This thermal energy builds up gas pressure that gets converted into mechanical power to propel the vehicle.
Electrical Power: Many power plants use thermal energy to produce electricity. For example, coal and nuclear plants heat water into steam that spins a turbine connected to a generator. Solar thermal plants focus sunlight to heat a fluid and create steam. Geothermal plants tap into underground thermal energy to produce steam as well.
In summary, thermal energy can be converted into usable mechanical power or electricity across many applications, making it a versatile and important form of energy in modern society.
Measuring Thermal Energy
Thermal energy can be precisely measured using various units and measurement tools. The main units used to quantify thermal energy are:
- Joules – The joule is the SI unit of energy, with 1 joule defined as the amount of energy required to produce 1 watt of power for 1 second.
- Calories – The calorie was originally defined as the amount of energy needed to raise 1 gram of water 1 degree Celsius. 1 calorie equals 4.184 joules.
- BTUs – The British Thermal Unit (BTU) is a traditional unit of heat still widely used today. 1 BTU is the amount of energy needed to raise 1 pound of water 1 degree Fahrenheit. 1 BTU equals 1055 joules.
Two important thermal properties used to measure heat capacity are specific heat and heat capacity:
- Specific Heat – The specific heat of a material is defined as the amount of heat needed to raise 1 kg of the substance 1 degree Celsius. Substances with higher specific heats require more thermal energy to change temperature.
- Heat Capacity – Heat capacity is the amount of heat needed to raise the temperature of an object by 1 degree Celsius. It depends on the mass, specific heat and change in temperature.
Key devices used to measure thermal energy and temperature include:
- Thermometers – Thermometers utilize the expansion of substances like mercury or alcohol to measure temperature changes.
- Calorimeters – Calorimeters allow measuring heat flow by heating a known mass of water. The temperature change indicates the amount of heat energy.
- Heat Flux Sensors – These sensors can precisely measure heat flow through a surface over time.
By quantifying thermal energy transfers with these tools and units, the principles of thermodynamics can be applied in science and engineering. Accurate measurement is key to understanding and controlling the role of heat in physical processes.
Thermal Energy Storage
Thermal energy can be stored for later use in a variety of materials and systems. One method is through phase change materials (PCMs) which absorb and release heat at certain temperatures. PCMs like ice, molten salts, and paraffin waxes can store significant amounts of thermal energy in their latent heat of fusion. Water tanks are another thermal storage medium, utilizing the heat capacity of water.
Thermal energy storage allows renewable energy sources like solar thermal power to store heat for electricity generation at night. It also facilitates load shifting to better match energy supply and demand. Thermal storage is used for heating and cooling of buildings, industrial processes, and vehicles like electric cars. Advanced phase change materials and storage system designs continue to improve the efficiency and capacity of thermal energy storage.
Thermal Energy and Heat
Although the terms are sometimes used interchangeably, there is a distinct difference between thermal energy and heat. Thermal energy refers to the total energy of all the molecules within a substance. This energy may include potential energy, kinetic energy, and interactions between molecules. Heat, on the other hand, is the transfer of thermal energy between substances. When two objects at different temperatures come into contact, thermal energy will transfer from the higher temperature object to the lower temperature object. This energy transfer is known as heat.
For example, a pot of water sitting on the stove contains a certain amount of thermal energy due to the motion and interactions of the water molecules. As the burner heats the pot, energy is transferred to the water, increasing its thermal energy. This transfer of energy from the burner to the water is heat. Once the water reaches the boiling point, no more heat can be transferred, but the water still contains thermal energy in the form of kinetic energy as the water molecules move vigorously.
So in summary, thermal energy refers to the total energy of all the molecules in a substance while heat refers specifically to the transfer of thermal energy between substances. Recognizing the difference between the two is important when discussing thermodynamic concepts and processes.
Thermal Energy and Temperature
Thermal energy and temperature are two related but distinct concepts. While they are closely linked, it is important to understand the key differences between them.
Thermal energy refers to the total kinetic energy of molecular motion in a substance. This molecular motion is directly related to the temperature of the substance. As thermal energy increases, molecular motion increases and temperature rises. Thermal energy is therefore a measure of the total energy contained in a system due to the motion of its molecules.
Temperature, on the other hand, is a measure of how hot or cold a substance is. More specifically, temperature is a measure of the average kinetic energy of molecular motion in a substance. Temperature indicates the direction of heat flow between two objects and provides a relative measure of how hot or cold an object is compared to a reference point.
While temperature and thermal energy are related, temperature provides a measure of intensity or concentration of thermal energy. An object may contain a high amount of thermal energy overall, but have a low temperature if the energy is spread over a large number of molecules. Similarly, a small object could have a high temperature if the thermal energy is concentrated in a relatively small number of rapidly vibrating molecules.
In summary, thermal energy refers to the total kinetic molecular energy in an object or system, while temperature measures the intensity or concentration of that thermal energy. Understanding the distinction between these two related concepts is key to accurately describing thermal physics.
Key Takeaways
Thermal energy, also known as heat energy, is absolutely a form of energy. It is the energy associated with the kinetic motion of atoms and molecules, and the potential energy stored in the bonds between atoms. Thermal energy can be transferred between objects through processes like conduction, convection, and radiation. Measuring thermal energy involves temperature, heat capacity, and transfer of heat. Thermal energy has many applications and implications in science, engineering, and everyday life. As a form of energy, thermal energy is essential to many systems and obeys the key principles that apply to all forms of energy. When studying energy, thermal energy and heat cannot be excluded. They play a fundamental role alongside other forms like chemical, mechanical, electrical, and nuclear energy.