Is Kinetic Energy Potential Or Kinetic?
Define kinetic and potential energy
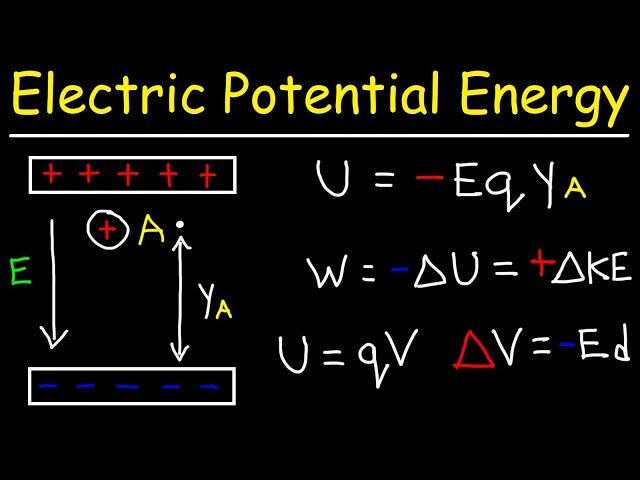
Kinetic energy is the energy of motion. It is defined as the work needed to accelerate a body of a given mass from rest to its current velocity. Kinetic energy depends on the mass and velocity of an object (Source 1). The formula for kinetic energy is:
EK = 1/2 mv2
Where m is mass and v is velocity. Some examples of kinetic energy are a bowling ball rolling down a lane or a person running.
Potential energy is stored energy that results from an object’s position or chemical composition. It is the energy possessed by an object by virtue of its position relative to others, stresses within itself, its electric charge, or other factors (Source 2). Potential energy can be released and turned into kinetic energy. Some examples are water held behind a dam or a book placed on a shelf.
Forms of kinetic and potential energy
Kinetic energy exists in several forms including vibrational, rotational, and translational kinetic energy. Vibrational kinetic energy relates to the vibration and oscillation of objects and systems. Molecules have vibrational kinetic energy from the movement of their atoms. Rotational kinetic energy refers to the rotation of an object around an axis. Examples include a spinning flywheel or the Earth rotating on its axis. Translational kinetic energy refers to the motion of objects from one location to another. A car driving down a road has translational kinetic energy.
Potential energy also exists in various forms including gravitational, elastic, chemical, and nuclear potential energy. Gravitational potential energy relates to height and the strength of gravity. When an object is lifted upwards, it gains gravitational potential energy. Elastic potential energy refers to objects that can be deformed, such as metal springs. When stretched or compressed, elastic objects store elastic potential energy. Chemical potential energy exists in the bonds between atoms in molecules and compounds. Strong bonds have high chemical potential energy that can be released during chemical reactions. Nuclear potential energy relates to the binding energy in the nucleus of atoms, which is released during nuclear fission and fusion reactions.
Relationship between kinetic and potential energy
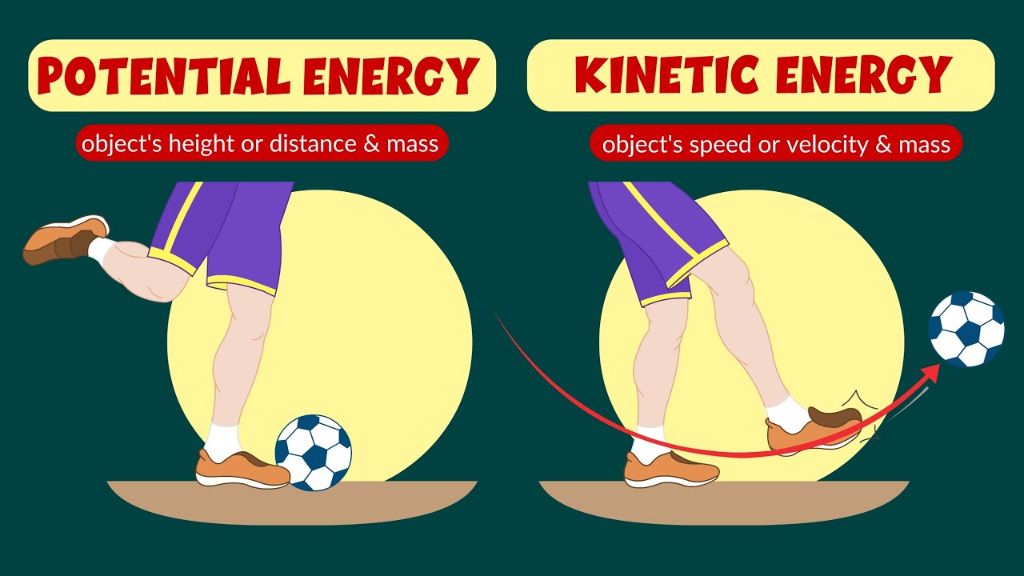
Potential and kinetic energy have an important relationship. Potential energy is stored energy in an object based on its position, shape or the arrangement of its parts. Common examples include gravitational potential energy from an object’s height, or elastic potential energy stored in a compressed spring.[1] Kinetic energy, on the other hand, is energy of motion, whether it is motion of the entire object, or motion of parts of the object. Kinetic energy depends on the mass and speed of the object, with more massive and faster objects having more kinetic energy.[2]
While kinetic energy and potential energy are fundamentally different types of energy, they can be interconverted. For example, when a ball is held above the ground, it has gravitational potential energy. When released, this potential energy is converted into kinetic energy as the ball accelerates towards the ground under gravity. The kinetic energy can then be converted back into gravitational potential energy if the ball bounces back upwards. However, at any given moment the ball’s energy is either kinetic (when moving) or potential (when stationary), it cannot be both simultaneously.
Examples to Illustrate the Difference
A classic example that illustrates the difference between kinetic and potential energy is a ball at the top of a ramp. When the ball is at rest at the top of the ramp, it has potential energy from its position relative to the Earth’s surface. According to Lesson: Kinetic vs. Potential Energy, “A ball at the top of a ramp has potential energy” (source). As the ball starts rolling down the ramp, the potential energy gets converted into kinetic energy, or energy of motion. What is an example of kinetic energy converted into potential energy? Explains that “A ball rolling down the ramp gains kinetic energy” (source). The ball accelerates as it rolls down the ramp, gaining more and more kinetic energy.
Other examples that demonstrate the difference include a book held up over the floor, which has potential energy, and the same book falling, which has kinetic energy. A stretched or compressed spring has potential energy, while a spring bouncing displays kinetic energy. In each case, there is a transfer between potential and kinetic energy.
Conservation of mechanical energy
The principle of conservation of mechanical energy states that in an isolated system that is only subjected to conservative forces, the total mechanical energy remains constant. Mechanical energy is the sum of a system’s kinetic energy and potential energy. In the absence of non-conservative forces like friction or air resistance, the total mechanical energy is conserved. This is known as the law of conservation of mechanical energy.
Kinetic energy is energy associated with motion and depends on the mass and speed of an object. Potential energy arises from an object’s position or shape and includes gravitational potential energy from an object’s height, elastic potential energy from deformation of an elastic object, and chemical potential energy from the nature and configuration of chemical bonds. The sum of all forms of potential and kinetic energy is mechanical energy.
For example, when a ball rolls down a hill, it loses gravitational potential energy but gains an equal amount of kinetic energy. As long as only conservative forces act, the mechanical energy before will equal the mechanical energy after. While energy can change forms, the total amount of mechanical energy remains fixed.
The conservation of mechanical energy is a fundamental principle in physics and has many important real-world applications in engineering and mechanics. It allows predicting system behavior and is used to improve efficiency and design.
Real world applications
Understanding kinetic and potential energy allows for the design of systems and devices that utilize the conversion between these two forms of energy. Some common examples include:
Rollercoasters are designed to maximize thrills by strategic conversions of gravitational potential energy into kinetic energy of motion. The potential energy builds as the coaster ascends the lift hill, converting into fast kinetic energy as it zooms downhill. The coaster’s speed boosts at the top of hills due to conservation of energy.
Springs and pendulums involve the interconversion of kinetic energy and elastic potential energy. A compressed spring contains stored elastic potential energy, which is converted into kinetic energy as the spring expands. The kinetic energy is then converted back into potential energy as the spring compresses again in the opposite direction. Pendulums follow a similar oscillating pattern between kinetic energy at the bottom of the swing, and gravitational potential energy at the top.
Hydroelectric dams use the potential energy of water stored in reservoirs, created by gravity acting on the water’s mass from higher elevation. As water is released through the dam and flows downhill through turbines, the potential energy converts into electricity from the kinetic energy of the moving water.
Batteries, fuel cells, and combustion engines involve chemical potential energy stored in the bonds of molecules like hydrocarbons and hydrogen. This chemical energy gets converted into kinetic energy of motion, electricity, or heat upon reaction. The resulting kinetic energy can power cars, generators, and many other devices.
Common misconceptions
There are some common misconceptions people have regarding kinetic and potential energy:
Many think kinetic energy is “live” or “active” energy, while objects at rest have no energy. This is not true, as stationary objects can have potential energy. As stated by the Energy Conceptions & Misconceptions report, “Non-moving objects have potential energy (sometimes called stored energy)” [1].
Related to this, people often don’t realize that potential energy is stored energy. As the Misconceptions – Energy resource explains, some believe “Gravitational potential energy depends only on the height of an object and kinetic energy only on the speed. The mass and gravitational field strength are not considered” [2]. In reality, an object’s potential energy depends on its mass and height in a gravitational field.
Understanding that stationary objects can possess stored potential energy is key to grasping kinetic vs potential energy.
Kinetic Energy in Physics and Chemistry
Kinetic energy in physics refers to the energy of motion of macroscopic objects like cars, balls, and planets. It depends on the mass and velocity of an object and is calculated using the equation Ek = 1/2mv2. In chemistry, kinetic energy refers to the energy associated with the motion of atoms and molecules. This is called molecular kinetic energy.
Molecular kinetic energy depends on the absolute temperature and results from the random motions of atoms and molecules. As temperature increases, molecular kinetic energy increases because the molecules move faster on average. Molecular collisions also depend on kinetic energy. Reactions proceed faster at higher temperatures because of the higher molecular kinetic energy.
Chemical reactions require a minimum input of energy called the activation energy to get started. This is the energy needed to distort bonds so that new bonds can form. Molecular kinetic energy provides this activation energy. At higher temperatures, more molecules have enough kinetic energy to reach the activation energy and react. So temperature increases the rate of chemical reactions by increasing molecular kinetic energy.
While related, kinetic energy in physics and chemistry have different implications. In physics, kinetic energy applies to large objects we can observe. In chemistry, it is a microscopic property of individual atoms and molecules that affects chemical reactivity. But both play an important role in their respective fields.
Importance and implications
Kinetic and potential energy are absolutely fundamental concepts in physics and chemistry, representing some of the most important forms of mechanical energy. Understanding the transformation between these two forms of energy is essential in mechanics, thermodynamics, and many other scientific fields. These energy concepts are key to analyzing how systems and processes work, and are crucial to designing efficient systems that minimize energy loss.
For example, potential energy can be stored and then converted to useful kinetic energy in a hydroelectric dam when water is released to spin turbines and generate electricity. Knowledge of potential and kinetic energy underpins numerous inventions and technologies central to modern civilization – from simple machines like levers and pulleys, to complex systems like internal combustion engines and rollercoasters. The study of kinetic and potential energy permeates all aspects of science and engineering.
On a theoretical level, the principle of the conservation of mechanical energy, which ties kinetic and potential energy together mathematically, is profoundly important. This law allows the total energy of isolated systems to be calculated and is the basis of dynamics and mechanics. Understanding kinetic and potential energy provides great insight into the physical world and is key to progress in fields like physics, chemistry, engineering, and more.
Summary
In summary, kinetic energy is the energy of motion while potential energy is stored energy based on an object’s position or arrangement (Potential and Kinetic Energy Explained | Education Overview). Kinetic energy depends on the mass and velocity of an object, whereas potential energy depends on factors like height, charge, stress, and more. Kinetic and potential energy can convert between each other, but the total mechanical energy remains constant according to the law of conservation of energy. For example, a ball held at a height has potential energy, which converts to kinetic energy as gravity accelerates it downward. When it hits the ground, the kinetic energy dissipates and is no longer present.
Energy is always conserved and can transfer between different forms like kinetic, potential, thermal, and more. But the total energy present before and after such transfers remains the same.