Is Chemical Potential Energy In Humans?
What is chemical potential energy?
Chemical potential energy is the energy stored in the chemical bonds of molecules. It is a form of potential energy that can be released as molecules undergo chemical reactions or changes in their atomic structure.
Some examples of chemical potential energy include:
- Energy stored in the molecular bonds of food, gasoline, batteries, and explosives
- Energy stored in ATP molecules that provide energy for cellular processes
- Energy stored in chemical reactants that can undergo reactions like combustion or metabolism
This chemical energy is stored within the structure of molecules. Atoms in molecules are held together by chemical bonds, which require energy to break. Strong bonds like those in carbon or hydrogen store more potential energy than weak bonds. The configuration of molecular bonds represents stored chemical energy that can be released to do work when those bonds are broken via chemical reactions.
Chemical potential energy in food
Food contains chemical potential energy in the form of macronutrients like carbohydrates, fats and proteins. When we eat and digest food, the large molecules of carbs, fats and proteins are broken down into smaller molecules through the process of digestion. This breakdown process releases the chemical potential energy that was stored in the bonds of the large molecules.
For example, complex carbohydrates like starch and glycogen are broken down into simple sugars like glucose. Fats are broken down into fatty acids and glycerol. Proteins are broken down into amino acids. This breakdown process requires energy input, but much more energy is released and becomes available for the body to use.
The chemical potential energy stored in the bonds of carbohydrates, fats and proteins originally came from plants through photosynthesis. Plants converted solar energy into stored chemical energy. When we eat plants, or animals that ate plants, we absorb that stored chemical potential energy for our own use.
Digestion converts chemical energy
Digestion is the process of breaking down large food molecules into smaller molecules that can be absorbed and used by the body. It begins in the mouth, where the mechanical action of chewing and the chemical action of enzymes in saliva start to break down food. The food then travels to the stomach, where strong acids and powerful enzymes continue the breakdown process.
In the small intestine, the majority of digestion occurs. Pancreatic enzymes break down carbohydrates, fats, and proteins into simple sugars, fatty acids, and amino acids. Bile from the liver emulsifies fats, turning them into tiny droplets that enzymes can easily access. At the end of the small intestine, the majority of molecules are small enough to be absorbed through the intestinal lining and into the bloodstream.
Digestive enzymes catalyze chemical reactions that break large molecules into smaller ones. This requires energy to break the chemical bonds holding the large molecules together. When these bonds break, the energy that was stored in them is released and can be used by the body.
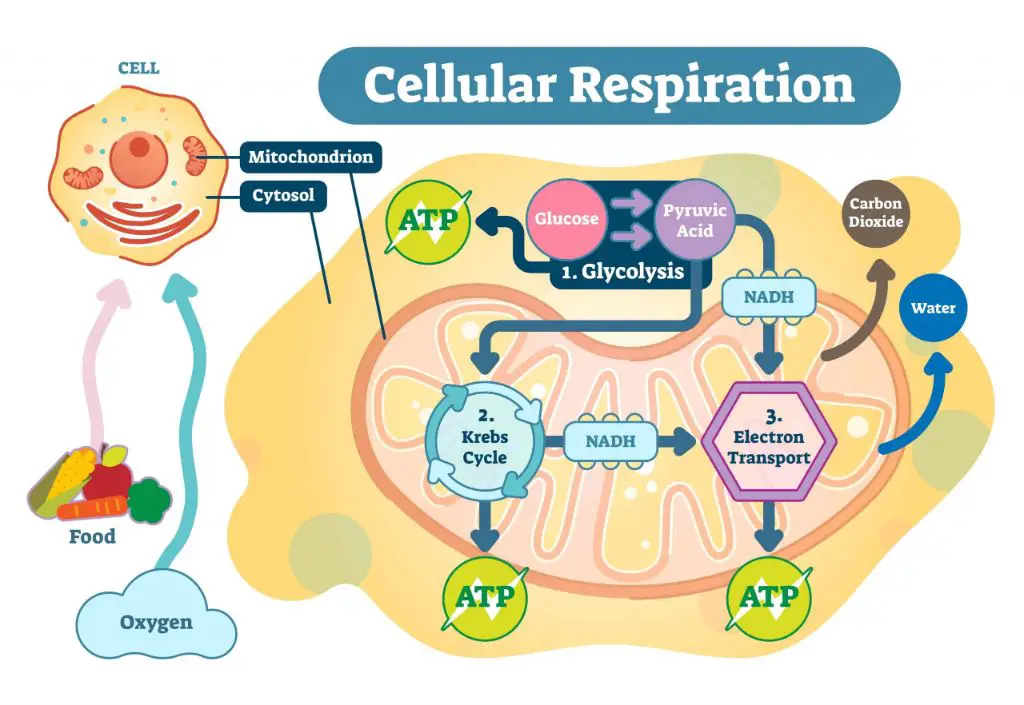
Cellular Respiration
Aerobic cellular respiration is the process that breaks down glucose and produces ATP. This multi-step process takes place in the mitochondria of cells and involves glycolysis, the Krebs cycle, and the electron transport chain. In glycolysis, glucose is broken down into pyruvate. The pyruvate enters the Krebs cycle where it is further broken down. This process generates electron carriers NADH and FADH2. These electron carriers provide energy for the electron transport chain to produce ATP.
The ATP generated from cellular respiration provides usable energy for the body. ATP powers many essential cellular processes and activities. Without the production of ATP from glucose, the body would not be able to function or sustain itself. The chemical energy stored in food is converted through cellular respiration into a form of energy that cells can readily use.
ATP powers cellular processes
ATP, or adenosine triphosphate, is a molecule that provides the main energy source for cells. It consists of an adenine base attached to a ribose sugar, with three phosphate groups attached. The chemical bonds between the phosphate groups contain high-energy electrons.
ATP powers cellular processes through ATP hydrolysis. This reaction breaks the bond between the second and third phosphate groups, releasing energy that can be harnessed for cellular work. The byproduct of ATP hydrolysis is ADP (adenosine diphosphate).
There are many critical cellular processes that rely on ATP as an energy source, including:
- Muscle contraction – ATP provides energy for muscle fibers to shorten and generate force.
- Nerve impulses – ATP powers ion pumps that establish neuron membrane potentials needed for nerve signaling.
- Biosynthesis – ATP provides energy to synthesize important biological molecules like proteins, lipids, and nucleic acids.
- Cell motility – ATP allows cells to move by powering cytoskeletal rearrangements and other mechanisms.
- Active transport – ATP energizes transport proteins that move ions and molecules against their gradients.
In this way, ATP acts as the key intersection between energy-releasing and energy-consuming reactions in cells. The ATP hydrolysis cycle is quickly regenerated by cellular respiration and photosynthesis.
Muscle contraction
Muscle contraction is powered by chemical energy stored in ATP molecules. Muscle cells contain myosin and actin filaments that slide past each other to create contraction. When a muscle needs to contract, ATP binds to the myosin head and provides the energy needed to change the myosin’s shape. The myosin head then connects to the actin filament and pulls it, shortening the muscle fiber. This process converts the chemical energy from ATP into mechanical work and movement. As ATP is hydrolyzed, the myosin disconnects from the actin, allowing the filaments to slide back to their relaxed positions. This cyclical process, fueled by ATP, is how muscles contract to enable body motions like walking, breathing, blinking, and more.
Nerve Impulses
Neurons use electrical signals to communicate with each other and with muscle or gland cells. An electrical impulse is generated when sodium ions rush into the neuron, changing its charge from negative to positive. This is called an action potential. After the impulse passes, ATP provides the energy to pump sodium back out of the cell and restore the neuron’s negative membrane potential.
Without sufficient ATP, neurons can’t fire properly and nerve impulses are disrupted. ATP allows neurons to repeatedly fire and carry electrical signals throughout the body. This enables everything from reflexes to thought processes and voluntary muscle movements.
Other uses of ATP
In addition to powering muscle contraction and nerve impulses, ATP is used for many other essential cellular processes. ATP provides the energy for:
- DNA and RNA synthesis – ATP is required for synthesizing the nucleic acids DNA and RNA. This is critical for storing genetic information and making proteins.
- Active transport of molecules – Cells use ATP to actively pump molecules across membranes against a concentration gradient. This maintains concentration differences between the inside and outside of cells.
- Motility of sperm, cilia, and flagella – The movement of sperm, cilia, and flagella is powered by ATP. These structures contain dynein motor proteins that use ATP hydrolysis to generate motion.
Through these and other processes, ATP powers many of the fundamental activities necessary for life at the cellular level.
Storing excess energy
The body has an ingenious way of storing excess glucose and fat for later use. When blood glucose levels rise after a meal, the pancreas secretes the hormone insulin, which signals cells to absorb glucose from the bloodstream. In the liver and muscles, excess glucose is converted into glycogen, a branched polysaccharide that serves as stored fuel. Glycogen molecules are densely packed to fit large amounts of glucose into a small space. Muscle cells can store enough glycogen to sustain activity for about 2 hours.
The body also stockpiles surplus calories in white adipose tissue, specialized cells that swell up into fat globules. Here, the excess glucose is converted into triglycerides, the storage form of fat. Stored fat provides reserve energy that can fuel the body’s needs when incoming calories are insufficient. On a typical diet, a person stores enough energy in glycogen and fat to provide weeks or months of energy if needed.
Conclusion
In summary, the chemical potential energy contained in the bonds of food molecules is utilized by cells through a multi-step process. First, digestion breaks down large food molecules into smaller components that can be absorbed. Then, through cellular respiration, cells extract the chemical energy stored in food and convert it into ATP. ATP serves as the molecular currency of energy in the body, powering many cellular processes.
ATP provides the immediate source of energy for muscle contraction, nerve impulses, protein synthesis, cell division, and the many other metabolic activities essential for life. Without the ability to generate ATP from food, our cells could not function. Therefore, the chemical potential energy contained in the foods we eat plays a critical role in powering the molecular machinery that keeps us alive.