Is Carbon In Every Living Thing?
Carbon is one of the most important chemical elements on Earth. It forms the basis of life as we know it and is present in every living thing on the planet. But is it truly found in every organism? This article will examine carbon’s vital role in biology and chemistry before exploring whether any exceptions to its necessity in life exist.
What is Carbon?
Carbon is a chemical element with the atomic number 6 and symbol C. It is a nonmetal with four valence electrons that can readily form covalent chemical bonds with many other elements.
Some key properties of carbon include:
- It is the sixth most abundant element in the universe and found in all known life forms.
- Carbon has four electrons available for bonding in its outer shell. This allows it to form diverse molecules by bonding with itself and other elements like hydrogen, oxygen, nitrogen, phosphorus, and sulfur.
- Carbon can form single, double, and triple covalent bonds. This enables the formation of long and complex chain molecules.
- Carbon bonds can be rigid, flexible, or have ring structures due to varying bond angles and bond lengths.
- Carbon compounds often have high melting and boiling points. They are also generally poor conductors of heat and electricity.
Role of Carbon in Life
Carbon is fundamental to life because it has a unique ability to form complex and stable molecules. Carbon can bond with up to four other atoms simultaneously, allowing it to form large, complex structures. It can also bond with itself to form long chains and ring structures, giving rise to the complex molecules that make up proteins, DNA, carbohydrates, and lipids – the fundamental components of all known life.
No other element has the ability to form such a diversity of molecules. The versatility of carbon makes it perfectly suited to form the wide range of compounds needed for biological processes. This versatility arises from carbon’s four outer electrons, allowing carbon to combine with many different elements, including hydrogen, oxygen, phosphorus and nitrogen.
Complex carbon-based molecules such as proteins and DNA store and transmit genetic information, provide structure to cells and organisms, catalyze metabolic reactions, and much more. Even simple carbon compounds play key roles, including carbon dioxide in photosynthesis and carbon skeletons in metabolism. The exceptional capacity of carbon to form molecular bonds and complexity underpins all life processes.
Carbon in Proteins and DNA
Proteins and DNA, two essential molecules for life, depend on carbon for their structure and function. Proteins are made up of amino acids, which contain carbon. The amino acids link together to form protein chains that fold into complex three-dimensional structures. The specific sequence of amino acids and the protein’s resulting shape determine its function. For example, carbon-containing amino acids make it possible for hemoglobin protein to bind oxygen in blood cells.
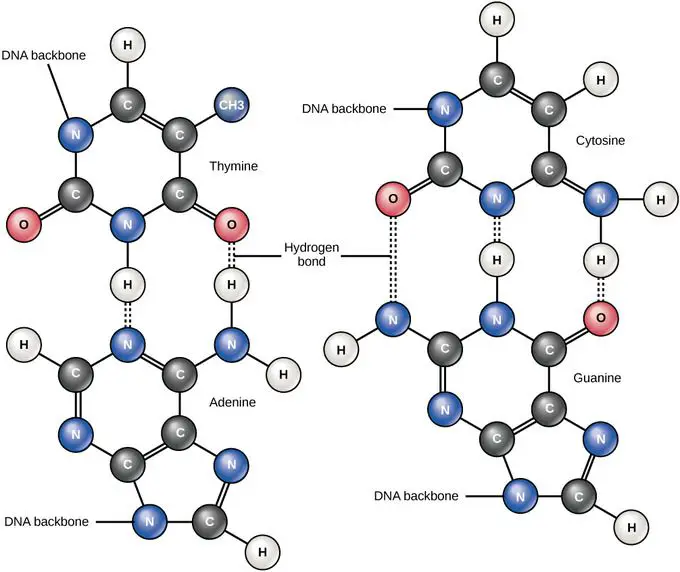
Likewise, carbon is a key part of the backbone of DNA structure. The two DNA strands are composed of repeating subunits of deoxyribose (a 5-carbon sugar molecule) and phosphates. Attached to the sugar-phosphate backbone are four nitrogenous bases – adenine, thymine, cytosine, and guanine. The sequence of these bases encodes genetic information. The carbon-containing deoxyribose and bases make DNA’s double helix structure possible, which is essential for storing, reading and duplicating genetic material.
In summary, proteins and DNA depend on carbon to form their essential structures. The ability of carbon to form multiple stable bonds is key to creating the complex shapes of proteins and the double-helix structure of DNA. Without carbon, these vital molecules of life would not exist.
Carbon in Cell Membranes
Cell membranes are one of the fundamental components of cells and are essential for life. They are composed primarily of a double layer of phospholipid molecules. Phospholipids have a hydrophilic (water-loving) phosphate head and two hydrophobic (water-fearing) hydrocarbon tails.
The hydrocarbon tails are long chains of carbon and hydrogen atoms. The carbon atoms form the structural backbone of the tails and provide shape, flexibility, and strength. The hydrocarbon tails are nonpolar and repel water, while the polar phosphate heads interact with water. This amphiphilic composition allows the phospholipid bilayer to form membranes.
The carbon atoms in the fatty acid tails connect to each other through covalent bonds, creating a stable structure. The flexibility of the hydrocarbon chains allows membranes to be fluid and dynamically changing. The nonpolar interactions between the hydrocarbon tails also help seal and isolate the interior of the cell.
In summary, the carbon atoms in the phospholipid bilayer enable cell membranes to selectively control what enters and exits the cell, provide structural support, and facilitate communication and transport across the membrane interface. Carbon is thus an essential element in this crucial component of cellular and organismal life.
Carbon in Sugars and Fats
Carbon forms the backbone of sugars and fats, two critical energy sources for life. Sugars like glucose and fructose consist of carbon atoms bonded with oxygen and hydrogen in ring or chain structures. The carbon backbone provides stability for the sugar molecule. Fats and oils also rely on carbon chains of fatty acids bonding to glycerol to create triglyceride molecules. The carbon-carbon bonds create long hydrophobic chains that give fats their water-repelling properties and ability to store energy.
Without carbon’s ability to form multiple stable bonds, sugars and fats would not exist. Carbon allows these molecules to take on complex shapes and properties that make them ideal for storage and transport of energy. In fact, the calorie content measured in foods comes directly from the chemical energy stored within the carbon bonds. Whether in the form of sugars circulating through the bloodstream or fats packed away in adipose tissue, the carbon backbone of these molecules provides living things with fuel to survive.
Carbon in the Human Body
Carbon makes up an astounding 18% of the human body’s mass. This high percentage reflects carbon’s central role in the chemistry of life. The main carbon-containing molecules in the human body include:
- Proteins – Proteins are large, complex molecules made up of amino acids. Amino acids contain carbon atoms bonded to hydrogen, oxygen, nitrogen and other elements.
- Nucleic acids like DNA and RNA – DNA and RNA are the key information storage molecules of the cell. Their backbone contains alternating units of phosphate and the 5-carbon sugar deoxyribose (in DNA) or ribose (in RNA).
- Lipids – Lipids like fats and oils are made up of fatty acids and glycerol. Both glycerol and fatty acids contain long chains of carbon atoms.
- Carbohydrates like starch and cellulose – Made up of sugar units, carbohydrates contain carbon, hydrogen and oxygen atoms.
In short, the fundamental chemistry of life relies heavily on carbon. Whether in proteins, lipids, nucleic acids or sugars, carbon forms the core structural scaffolding of important biological molecules.
Carbon in Plants and Animals
Carbon makes up the majority of plant and animal mass. On average, dry plant matter is around 45% carbon by mass. This carbon is found in many organic compounds that make up plant cell walls, fibers, proteins, nucleic acids, sugars, and lipids. Key carbon compounds in plants include cellulose, lignin, proteins, DNA, starch, and fats.
Animals have a similar carbon composition, with human dry body mass containing around 18% carbon. This carbon is present in proteins, DNA, fats, carbohydrates, and other biomolecules that enable animal life. Key carbon compounds in animals include collagen, keratin, melanin, carbohydrates, fatty acids, cholesterol, and phospholipids in cell membranes.
Without the element carbon and its ability to form diverse organic molecules, plants and animals would not exist in the way we know them. The fundamental molecules of life, including DNA, RNA, proteins, lipids, and sugars, all contain carbon as an essential building block.
Exceptions to Carbon-Based Life
While most life on Earth is carbon-based, there are a few potential exceptions. Silicon-based life forms have been hypothesised as an alternative biochemical system, as silicon has properties similar to carbon and also forms complex molecules. However, carbon is still essential even in hypothetical non-carbon-based life due to its unique chemical properties. Carbon can form stable bonds with many elements, allowing it to serve as the backbone of complex molecules like proteins, DNA, sugars and fats that make up living organisms. The versatility of carbon compounds likely makes carbon indispensable to life throughout the universe. Even if life is based on a different element, carbon would likely still play a fundamental role.
Another hypothetical exception is life with an “arsenic biochemistry”. In 2010, NASA researchers controversially claimed they had discovered a bacterium named GFAJ-1 that could use arsenic in place of phosphorus, which is essential for DNA, ATP and phospholipids in standard life. However, most evidence indicates GFAJ-1 does require phosphorus and incorporates arsenic in a non-essential way. So again, carbon remains essential even in organisms that may metabolize arsenic.
While silicon or arsenic based life is theoretically possible, carbon’s unique propensity for complex molecule formation makes it indispensable for life as we know it on Earth and likely throughout the cosmos. Even in speculative non-carbon-based biochemistries, carbon and its compounds are still likely essential in some capacity.
Conclusion
We’ve seen that carbon is essential for all known life on Earth. Carbon’s ability to form diverse molecular structures allows it to form the complex molecules necessary for life – including proteins, DNA, cell membranes, and more. From the carbon in our own bodies to the carbon that makes up all plants and animals, this versatile element enables life as we know it.
Carbon’s unique properties make it perfectly suited for the chemistry of life. While other elements contribute, carbon forms the fundamental backbone of the molecules that living things depend on. Whether something is made of skin, wood, or stone – if it’s alive, it’s made of carbon. Carbon’s central role and flexibility is why it’s found in every living thing on the planet.