Is All Energy Just Kinetic?
Energy is defined as the ability to do work or produce heat. Energy exists in many forms such as kinetic, potential, thermal, chemical, nuclear and more (Energy Definition & Meaning). Kinetic energy, specifically, is the energy associated with motion. An object that is moving has kinetic energy. The amount of kinetic energy depends on the object’s mass and velocity. For example, a bowling ball rolling down a lane has more kinetic energy than a ping pong ball rolling down the same lane because the bowling ball has more mass. Kinetic energy is one of the many forms that energy can take.
Forms of Energy
There are several different forms of energy that exist in the universe. Here are some of the main forms:
Potential Energy
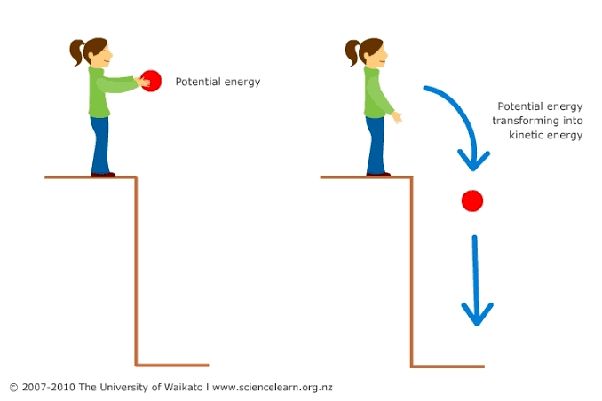
Potential energy is the stored energy an object has due to its position or state. For example, the energy stored in a spring that is compressed or stretched is potential energy [1].
Kinetic Energy
Kinetic energy is the energy an object possesses due to its motion. The faster an object moves, the more kinetic energy it possesses. For example, a moving car has kinetic energy [1].
Thermal Energy
Thermal energy arises from the random motion of molecules and atoms. The greater the molecular motion, the higher the thermal energy. Heat is the transfer of thermal energy from one object or system to another [1].
Chemical Energy
Chemical energy is the potential energy stored in the bonds between atoms that make up molecules. Chemical energy can be released during chemical reactions to produce thermal energy [1].
Nuclear Energy
Nuclear energy comes from the splitting (fission) or merging (fusion) of atomic nuclei. Nuclear power plants use nuclear fission to generate electricity [1].
Electrical Energy
Electrical energy results from the movement of electrically charged particles. It is a secondary form of energy produced from the conversion of other forms of energy [1].
Radiant Energy
Radiant energy is electromagnetic energy that travels in transverse waves, such as visible light, ultraviolet radiation, and X-rays. It can transfer energy from one location to another [1].
Kinetic Energy
Kinetic energy is energy of motion (Britannica, 2023). An object or particle has kinetic energy because of its motion. The amount of kinetic energy depends on the mass and velocity of the moving object (Khan Academy, 2023). The faster an object moves, the more kinetic energy it possesses. Even very small objects like atoms and subatomic particles have kinetic energy if they are moving (TechTarget, 2023).
The kinetic energy of an object is directly proportional to its mass. Doubling the mass of an object doubles its kinetic energy at the same velocity. Kinetic energy is also directly proportional to the square of the velocity – if the velocity doubles, the kinetic energy increases by a factor of four. This relationship is described in the kinetic energy formula:
Ek = 1/2 mv2
Where Ek is kinetic energy, m is mass, and v is velocity. According to this formula, an increase in either mass or velocity results in an exponential increase in kinetic energy.
Potential Energy
Potential energy is a form of stored energy that exists by virtue of an object’s position or configuration in a field of force. An object can store potential energy based on its relative position to another object through gravitational, magnetic, or electrostatic forces (source). For example, a rock held at a height above the ground contains gravitational potential energy due to the force of gravity acting on its mass. If the rock falls, this potential energy gets converted into kinetic energy as work is done against the gravitational force during the falling motion. Other examples are a compressed spring storing mechanical potential energy, or a capacitor storing electric potential energy in the electric field between its plates.
The key aspect that distinguishes potential energy from kinetic energy is that it is system dependent. Specifically, the amount of potential energy depends on the configuration of the system, not just the properties of a single object. Changes to the relative positions of objects in the system can change the total potential energy. Overall, potential energy provides a useful framework for analyzing mechanical systems across physics and engineering.
Thermal Energy
Thermal energy refers to the internal energy contained within a system that is responsible for its temperature (https://www.khanacademy.org/science/physics/work-and-energy/work-and-energy-tutorial/a/what-is-thermal-energy). It is the energy associated with the random motions of the molecules and atoms of a substance. The hotter a substance is, the faster its molecules vibrate and move. Thermal energy comes from the kinetic energy of these moving particles.
When there is a temperature difference between two objects or systems, heat flows from the hotter to the colder system as thermal energy is transferred. This flow of heat changes the internal energy of each system. The internal energy of the hotter system decreases while the internal energy of the colder system increases, causing its temperature to rise.
Common examples of thermal energy include the energy in hot water that can be used for cooking or bathing, the energy that allows an ice cube to melt, or the energy that keeps homes warm through heating systems (https://www.solarschools.net/knowledge-bank/energy/types/thermal). Thermal energy is an important form of energy in everyday life.
Chemical Energy
Chemical energy is energy stored in the bonds between atoms and molecules. It is the energy that holds these particles together. Chemical energy can be converted into other forms of energy when chemical reactions occur. For example, when a fuel like gasoline is burned, chemical energy stored in the gasoline’s molecular bonds is converted into heat and light energy.
Chemical energy is fundamentally derived from the atoms and molecules that make up a substance. Different substances have different amounts of energy stored in their chemical bonds based on the structure and type of atoms. Substances like fossil fuels, foods, and batteries contain a lot of stored chemical energy that can be released through chemical reactions.
The amount of chemical energy stored in a substance can be calculated from the number and types of bonds it contains. Strong chemical bonds like ionic, covalent, and metallic bonds contain more chemical energy than weak bonds like hydrogen bonds and van der Waals forces. Thus, the types of molecules and compounds influence the energy content.
Chemical energy drives many biological processes. In the human body, chemical energy derived from food molecules is converted through metabolic chemical reactions into energy forms needed to fuel the body. Chemical energy is also converted into thermal and mechanical energy in combustion engines to power transportation.
Nuclear Energy
Nuclear energy comes from the splitting of uranium atoms in a process called nuclear fission (1). During nuclear fission, a neutron collides with a uranium atom causing it to split into lighter atoms and release a large amount of energy in the form of heat (1, 2). The heat is used to boil water into steam that spins a turbine to generate electricity.
Nuclear power plants use uranium as fuel because its atoms are easily split apart (2). The energy released by uranium is over a million times greater than that of other fossil fuels. About 11% of the world’s electricity is generated by about 440 nuclear reactors (3).
Another form of nuclear energy comes from the combining of hydrogen atoms into helium, called nuclear fusion (2). This is the process that powers the sun and stars. Fusion releases an even larger amount of energy than fission. Scientists are still working to control nuclear fusion for energy production (3).
Overall, nuclear energy provides a clean energy source that does not produce air pollution or carbon dioxide. However, there are concerns over the safe disposal of radioactive waste products and possible nuclear accidents.
Electrical Energy
Electrical energy is the energy derived from the movement of electric charges. It is a form of kinetic energy, as the motion of electrons produces an electric current which can be harnessed to perform work. Electrical energy exists whenever charge is in motion. The greater the electric current, the greater the electrical energy.
Electrical energy results from the flow of electrons through a conductor. This flow of charges is referred to as an electric current. Electrical energy can be generated through various methods, such as batteries, generators, solar cells, etc. It is an extremely useful form of energy that allows electricity to power homes, appliances, factories, transportation, and communications. Almost all the modern amenities we enjoy involve the use of electrical energy.
Radiant Energy
Radiant energy is the energy carried by electromagnetic waves [1]. Electromagnetic waves are able to travel through space and do not require a medium like air or water. Examples of electromagnetic waves include visible light, ultraviolet light, infrared radiation, radio waves, and X-rays. Radiant energy can be described as either a wave phenomenon or as particles called photons. As a wave, it consists of oscillating electric and magnetic fields and moves at the speed of light. As photons, radiant energy exhibits particle properties of carrying energy and momentum [2].
Radiant energy is produced when charged particles are accelerated. For example, a radio antenna produces radiant energy in the form of radio waves when electrons in it are accelerated by a changing electrical current. The sun produces radiant energy in the form of visible light by accelerating charged particles during nuclear fusion reactions [3]. Radiant energy obeys the inverse square law – its intensity decreases proportional to the square of the distance from its source. This energy can be transmitted, absorbed, or reflected when interacting with matter.
[1] https://www.techtarget.com/whatis/definition/radiant-energy
[2] https://www.solarschools.net/knowledge-bank/energy/types/radiant
[3] https://www.repsol.com/en/energy-and-the-future/future-of-the-world/radiant-energy/index.cshtml
Conclusion
In summary, while kinetic energy is an important form of energy resulting from motion, it is not the only form of energy. Other forms of energy exist, including potential energy, thermal energy, chemical energy, nuclear energy, electrical energy, and radiant energy. These other types of energy can be converted into kinetic energy and vice versa, but they have their own unique properties and sources as well. Kinetic energy provides a useful framework for understanding many energetic processes, but it does not encompass all manifestations of energy in the world around us.
Energy comes in many forms beyond just kinetic energy. By exploring the different types of potential, thermal, chemical, nuclear, electrical, and radiant energy, we gain a fuller understanding of the diverse energetic phenomena in the universe. While motion and kinetics underpin many energetic processes, the full picture requires examining energy more holistically across its various manifestations.