Is A Temperature Difference Potential Or Kinetic Energy?
Defining Potential and Kinetic Energy
Potential energy is stored energy that an object has due to its position or state. For example, a ball held at a height above the ground has gravitational potential energy due to gravity’s effect on its elevated position. Similarly, a compressed spring has elastic potential energy due to its compressed state. In general, potential energy represents work that can be done by a system because of its arrangement.
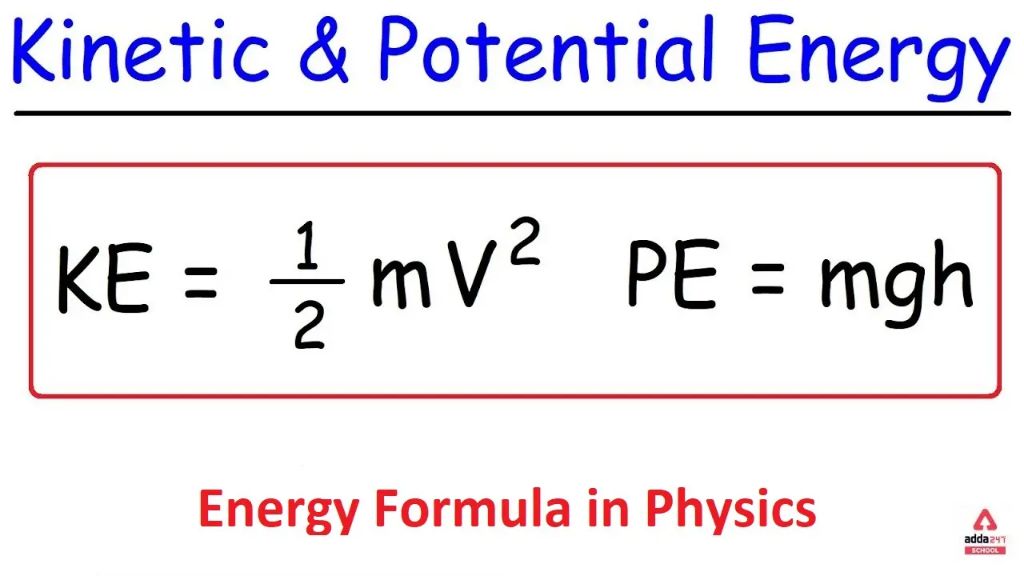
Kinetic energy is energy associated with motion. A moving object has kinetic energy equal to one-half its mass times its velocity squared. For instance, a baseball thrown at high speed has a large amount of kinetic energy that can be transferred upon impact to break a window. Kinetic energy can also refer to the movement of atoms and molecules; thermal energy is the kinetic energy associated with this microscopic motion.
Temperature as a Measure of Kinetic Energy
Temperature is a direct measure of the average kinetic energy of molecules within a substance. Kinetic energy is the energy of motion – the faster molecules move and vibrate, the more kinetic energy they possess. When heat is added to a substance, it causes the molecules to move and vibrate faster, gaining more kinetic energy. This increase in molecular motion and vibrations manifests itself as a rise in temperature.
On a microscopic scale, increasing the temperature of a substance like water causes the water molecules to move faster and bump into each other more forcefully. The more kinetic energy the molecules have from their accelerated motion, the higher the temperature reading. This is why heating water on a stove increases its temperature – the burner adds heat which gets converted into greater molecular kinetic energy.
We can measure this increase in kinetic energy using a thermometer. As the average molecular kinetic energy rises, the liquid within the thermometer expands, providing a quantifiable temperature reading. This allows us to correlate temperature values to the underlying kinetic energy at the molecular level. Essentially, temperature serves as a representation and measure of molecular kinetic energy.
Thermal Energy Transfer and Temperature Difference
When two objects are at different temperatures, heat will spontaneously flow from the hotter object to the colder object. This occurs because temperature is a measure of the average kinetic energy of molecules in a substance. At higher temperatures, molecules vibrate and move more rapidly, carrying more kinetic energy. When a hot and cold object come into contact, the faster moving molecules from the hotter object collide with the slower molecules of the colder object, transferring some of their kinetic energy and speeding up the motion of the colder molecules. This process continues until thermal equilibrium is reached and both objects are at the same temperature.
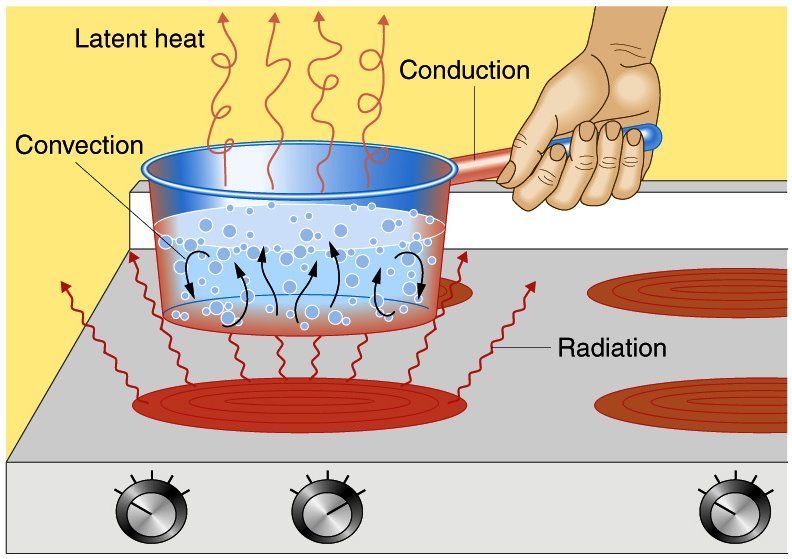
The greater the temperature difference between two objects, the faster the rate of heat transfer between them. This is because a larger temperature gradient provides more driving force to move heat from the high temperature region to the low temperature region. Thermal energy will always spontaneously disperse from hot to cold until thermal equilibrium is achieved. Therefore, the magnitude of the temperature difference is directly related to the potential for heat transfer. Large temperature differences have a high potential for driving vigorous heat transfer, while small temperature differences have lower potential.
Relating Temperature Difference to Potential Energy
Temperature difference between two systems represents a potential for spontaneous heat transfer, similar to gravitational potential energy in classical mechanics. Just as an object at a higher gravitational potential has the capacity to do work in falling, a system at a higher temperature has the capacity to transfer heat spontaneously to a lower temperature system. This potential exists by virtue of the temperature difference alone.
Gravitational potential energy is defined as U = mgh, where m is the object’s mass, g is the gravitational acceleration, and h is the height above a reference level. An object at rest at a height h has gravitational potential energy that can be converted into kinetic energy in falling. The greater the height, the greater the potential energy.
Analogously, the potential for heat transfer depends on the temperature difference between two systems. The greater the temperature difference ΔT between the systems, the greater the potential for spontaneous heat transfer from the hotter to the colder system. This potential exists even if no heat is currently being transferred, just as gravitational potential exists even if the object is at rest.
In summary, temperature difference represents a potential for spontaneous thermal energy transfer, in the same way that height difference represents a potential for mechanical energy transfer. This potential is quantified by the magnitude of the temperature difference ΔT.
Examples and Applications
Temperature differences drive energy transfers in many everyday scenarios. For instance, when you boil water on a stove, the temperature difference between the hot stove burner and the cooler water causes heat transfer from the burner to the water. As the water absorbs this heat, its temperature rises until it reaches boiling point.
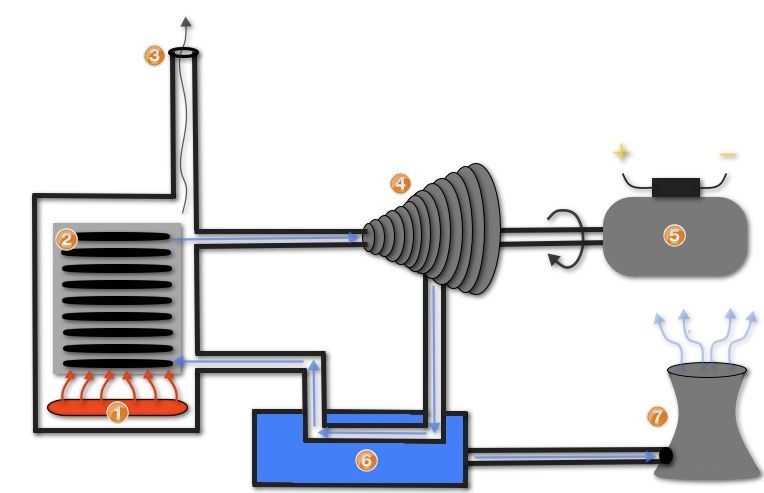
On a larger scale, power plants use temperature differences to generate electricity. In coal power plants, coal is burned to heat water and produce high-pressure steam. This steam then flows through a turbine, which spins to generate mechanical power. The turbine spins an electrical generator, producing electricity. The temperature difference between the hot steam and cooler condenser creates the potential energy that drives the turbine.
Similarly, nuclear power plants use nuclear fission to heat water. The resulting steam flows through a turbine, generating electricity in the same way as coal plants. The temperature difference enables these heat engines to convert thermal energy into mechanical work.
In refrigerators and air conditioners, the cycle is reversed. Work is done by a compressor to move refrigerant between a cold coil and a hot condenser coil. This enables heat transfer from a cold interior space to a warmer external environment, driven by temperature differences between the coils.
Thermodynamics Laws
The laws of thermodynamics govern the transfer of energy and explain how temperature differences relate to energy potential. The zeroth law of thermodynamics states that if two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. This law establishes the concept of temperature and enables the measurement of temperature.
The first law of thermodynamics, also known as the law of conservation of energy, states that energy can neither be created nor destroyed, only transformed from one form to another. In the context of temperature difference, the first law says that a temperature difference represents potential energy that can be transformed into heat and work.
The second law of thermodynamics states that the entropy of an isolated system always increases over time. Entropy is a measure of disorder, so the second law implies that isolated systems tend to evolve toward thermal equilibrium and a uniform temperature distribution. A temperature difference represents a non-equilibrium state with lower entropy, meaning there is potential energy available to be transferred as the system moves toward equilibrium.
In summary, the laws of thermodynamics govern how temperature differences and energy transfers are related. Temperature gradients represent potential energy that can drive spontaneous thermal energy transfers. The laws provide the foundation for quantifying temperature difference as a form of potential energy.
Quantifying Temperature Difference Potential
The potential energy stored in a temperature difference can be quantified using thermodynamic calculations. The key formula is:
U = m*c*ΔT
Where:
- U is the potential energy (in Joules)
- m is the mass of the substance (in kg)
- c is the specific heat capacity of the substance (in J/kg*K)
- ΔT is the temperature difference (in Kelvin)
For example, consider 1 kg of water with a temperature difference of 10°C (or 10 K). The specific heat capacity of water is 4186 J/kg*K.
Plugging this into the formula:
U = 1 kg * 4186 J/kg*K * 10 K
U = 41,860 J
So a 10°C temperature difference in 1 kg of water results in 41,860 J of potential energy. This calculation can be applied to any substance to quantify the potential energy stored in a given temperature difference.
Comparing to Other Potentials
Temperature difference potential can be compared to other common forms of potential energy like gravitational potential energy and electric potential energy. Just as an object can have gravitational potential energy due to its position in a gravitational field, or electric potential energy due to its position in an electric field, a system can have thermal potential energy due to a difference in temperature.
For example, consider a rock held at a height above the ground. It has gravitational potential energy that can be turned into kinetic energy if released. Similarly, consider two objects at different temperatures in thermal contact. The hotter object has a thermal potential to transfer heat to the colder object. This potential can be turned into kinetic heat transfer between the objects if allowed to come into contact.
While gravitational and electric potentials are dependent on mass and charge respectively, thermal potential depends on temperature. All represent forms of latent potential energies that can be transformed into other active forms of energy under the right conditions. Quantitatively, the thermal potential energy between two systems due to a temperature difference can be calculated from thermodynamic principles, just like how gravitational and electric potential energies can be calculated from their respective governing equations.
Historical Context
The foundations of thermodynamics and our modern understanding of temperature difference as a form of potential energy emerged in the 19th century through the pioneering work of scientists such as Sadi Carnot, James Joule, Lord Kelvin, and Rudolf Clausius.
In 1824, Sadi Carnot published his groundbreaking analysis of the efficiency of steam engines, laying the foundations for the second law of thermodynamics. Carnot recognized that heat could be used to do work, and that there was a maximum possible efficiency for heat engines based on the temperature difference between the hot and cold reservoirs.
Building on Carnot’s insights, in the 1840s James Joule experimentally showed the equivalence between heat and other forms of energy. This helped establish the principle of conservation of energy and the idea that heat and work were interconvertible.
Around the same time, Lord Kelvin helped develop the absolute temperature scale, proposing the use of degrees Celsius and defining “absolute zero”. This allowed more precise quantification and measurement of temperature differences.
In 1850, Rudolf Clausius coined the term “entropy” and formulated the second law of thermodynamics. Clausius showed that heat flows spontaneously from hotter to colder bodies, and that this transfer depends on the temperature difference. This further cemented the link between temperature gradients and potential energy.
Through the trailblazing work of these scientists, by the late 19th century the foundations of thermodynamics and the relationship between temperature difference and potential energy were well established.
Conclusion
As we have learned, temperature is a direct measure of the average kinetic energy of molecules in a substance. A temperature difference between two systems represents potential energy, as heat will spontaneously flow from higher temperature to lower temperature if they are allowed to interact. This potential energy can be harnessed to perform useful work. The laws of thermodynamics govern how this energy transfer occurs. While temperature difference potential is distinct from other common forms of potential energy like gravitational or electric potential, it still obeys similar principles. Understanding the relationship between temperature, heat, work, and energy is key to many applications in science and engineering.
In summary, a temperature difference does indeed represent a form of potential energy. This potential can drive heat engines and allow useful work to be extracted from thermal systems. Quantifying and properly utilizing temperature difference potentials has been an important breakthrough in the history of thermodynamics.