In Which Chemical Energy Is Transformed Into Electric Energy In These Cells?
Cells are the basic structural and functional units of all living organisms. They contain organelles and biochemical structures that allow them to obtain and utilize energy. There are two main types of energy that cells require: chemical energy and electrical energy.
Chemical energy is the energy stored within the bonds of molecules. Cells get this energy by breaking down fuel molecules like glucose during cellular respiration. The chemical energy stored within glucose can be converted into other forms of energy that the cell can use.
Electrical energy involves the movement of charged particles, like protons and electrons. This form of energy is crucial for cells to carry out processes like transferring signals and transporting molecules across membranes. Electrical gradients are generated across membranes in certain cellular organelles, creating voltage that cells harness.
The goal of this content is to explore how cells are able to transform the chemical energy from food molecules into electrical energy that the cell can utilize. This energy conversion takes place through a series of reactions and processes within special organelles.
Chemical Energy Storage
The transformation of chemical energy into electrical energy takes place through a series of chemical reactions that store energy in the form of ATP molecules. ATP, or adenosine triphosphate, is the main energy currency of cells and serves as a convenient way to store and transport chemical energy within an organism.
ATP is composed of an adenine nucleobase, a ribose sugar, and three phosphate groups. The bonds between the phosphate groups contain high-energy electrons that can be used to power cellular reactions and processes. When the terminal phosphate bond is broken through a process called hydrolysis, energy is released and used to perform work while ATP becomes ADP (adenosine diphosphate). The regeneration of ATP from ADP is key to continually supplying cells with energy.
Glycolysis
Glycolysis is the first step in cellular respiration, where glucose is broken down anaerobically into pyruvate to produce a net gain of 2 ATP molecules. This multi-step reaction occurs in the cytoplasm of the cell and does not require oxygen.
The glycolysis pathway can be summarized in two main phases:
1. Energy Investment Phase: ATP is invested to phosphorylate glucose into glucose-6-phosphate. This initial investment helps destabilize the glucose molecule so it can be split in the next steps.
2. Energy Generation Phase: Glucose-6-phosphate is further split to create 2 pyruvate molecules, generating a net gain of 2 ATP and 2 NADH molecules. This constitutes the energy “payoff” phase.
The end products of glycolysis, ATP and NADH, can be used directly for energy or fed into the next steps of cellular respiration to extract more energy. Importantly, glycolysis generates ATP without the use of oxygen, allowing cells to produce energy even under anaerobic conditions.
Krebs Cycle
The Krebs cycle, also known as the citric acid cycle or tricarboxylic acid (TCA) cycle, is the second stage of cellular respiration. This cycle occurs in the mitochondrial matrix and further breaks down the products from glycolysis.
In the Krebs cycle, the acetyl group from acetyl CoA is attached to a four-carbon molecule called oxaloacetate to form citrate. Citrate then goes through a series of chemical reactions, being converted into different molecules like isocitrate, α-ketoglutarate, succinyl CoA, succinate, fumarate, malate, and finally back to oxaloacetate.
In these reactions, the acetyl group is further oxidized, producing high-energy electrons that are fed into the electron transport chain. Each turn of the Krebs cycle produces 2 CO2 molecules, 3 NADH, 1 FADH2, and 1 ATP or GTP molecule. This further breakdown of the byproducts from glycolysis allows for more production of ATP.
Electron Transport Chain
The electron transport chain (ETC) is a series of protein complexes and electron carriers located in the inner mitochondrial membrane that shuttles electrons from electron donors to electron acceptors via redox reactions. The electron transport chain couples electron transfer between electron donors and acceptors with the transfer of protons (H+) across the membrane, generating an electrochemical proton gradient.
In the ETC, electrons are passed through a series of electron carriers, with each carrier having a more positive redox potential than the previous one. At each step, the energy released from these redox reactions is used to pump protons from the mitochondrial matrix into the intermembrane space. This creates an electrochemical gradient across the inner mitochondrial membrane, which drives the synthesis of ATP.
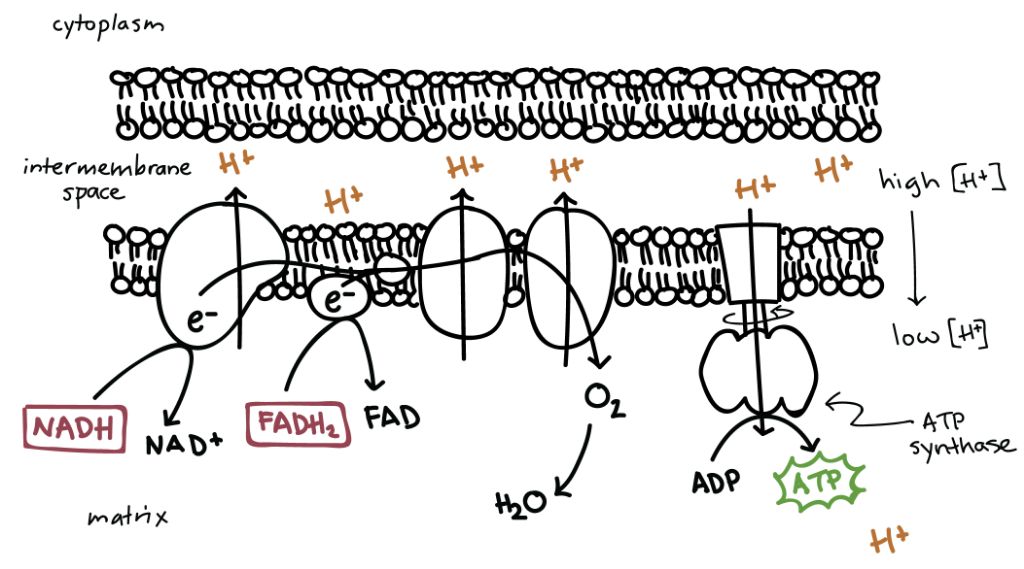
Some key steps in the ETC are:
- NADH and FADH2 donate electrons to Complex I and Complex II respectively.
- The electrons are then passed to ubiquinone (Coenzyme Q), which carries the electrons to Complex III.
- Complex III transfers the electrons to Cytochrome c, another mobile electron carrier.
- Cytochrome c takes the electrons to Complex IV, where the electrons are used to reduce molecular oxygen to water.
As the electrons move through the ETC, protons are pumped from the matrix to the intermembrane space at Complexes I, III and IV. This establishes both a pH gradient and an electrical gradient across the inner membrane, which comprises the proton-motive force used to drive ATP synthesis.
Oxidative Phosphorylation
Oxidative phosphorylation is the process that converts the energy stored in food to a chemical form that cells can use. This chemical energy is stored in a molecule called adenosine triphosphate (ATP).
Oxidative phosphorylation takes place in the inner membrane of the mitochondria. As electrons move through the electron transport chain, hydrogen ions (H+) are pumped across the inner mitochondrial membrane, creating an electrochemical gradient. This gradient stores potential energy, like water held behind a dam.
The enzyme ATP synthase acts as a channel protein that allows H+ to flow back across the membrane. As H+ flows down its gradient through ATP synthase, the enzyme harnesses the energy to phosphorylate adenosine diphosphate (ADP), adding a phosphate group to produce ATP. This process of harnessing energy from H+ to synthesize ATP is called chemiosmosis.
In this way, the energy stored in food is gradually converted into a form that cells can utilize through a series of redox reactions and phosphorylation steps. The whole process results in the synthesis of many ATP molecules that provide chemical energy for cellular processes.
Chemiosmosis
Chemiosmosis is the process that utilizes the electrochemical gradient created by the electron transport chain to drive ATP synthesis. This process takes place in the inner mitochondrial membrane of eukaryotes and the plasma membrane of prokaryotes.
As electrons move through the electron transport chain, protons (H+) are pumped across the membrane from the mitochondrial matrix into the intermembrane space, creating an electrochemical proton gradient. This gradient consists of both a pH gradient (a difference in H+ concentration across the membrane) and an electrical gradient (a difference in charge across the membrane).
The protein ATP synthase contains a channel that allows H+ to flow down their electrochemical gradient, from the intermembrane space back into the matrix. This flow of protons causes components of ATP synthase to spin, providing the energy needed to synthesize ATP from ADP and inorganic phosphate.
Because an electrochemical proton gradient is required for ATP synthesis, this process is called chemiosmosis. Chemiosmosis couples electron transport to ATP production, allowing the energy from redox reactions to be harnessed into a usable form of chemical energy.
Mitochondria
The mitochondria are specialized organelles found in the cytoplasm of eukaryotic cells. They are often referred to as the “powerhouses” of the cell because they generate most of the cell’s supply of adenosine triphosphate (ATP), the molecule that provides energy to power cellular processes.
ATP is produced in the mitochondria through a process called cellular respiration. This involves the oxidation of nutrients like glucose to release energy, which is used to synthesize ATP. There are three main stages of cellular respiration that occur in the mitochondria: glycolysis, the Krebs cycle, and oxidative phosphorylation.
In glycolysis, glucose is broken down into pyruvate and a small amount of ATP is generated. The pyruvate then enters the Krebs cycle where it is further oxidized to release more energy carriers like NADH and FADH2. These energy carriers bring the energy from the Krebs cycle to the electron transport chain, located on the inner membrane of the mitochondria. Here, their energy is used to pump protons across the membrane, generating an electrochemical gradient. This gradient powers ATP synthase to produce ATP in a process called oxidative phosphorylation. This final stage produces over 30 molecules of ATP per glucose molecule.
In summary, the mitochondria generate the vast majority of a cell’s ATP supply through cellular respiration and oxidative phosphorylation. This makes them a crucial organelle for providing cells with the chemical energy they need for active processes.
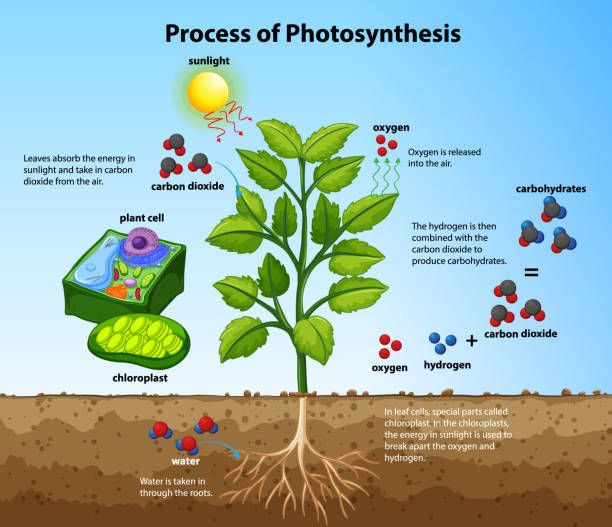
Chloroplasts
Chloroplasts are organelles found in plant cells that conduct photosynthesis. Photosynthesis is the process plants use to convert light energy from the sun into chemical energy in the form of glucose. This process takes place in the thylakoid membranes inside chloroplasts.
The thylakoid membranes contain chlorophyll, a green pigment that absorbs sunlight. When sunlight hits the chlorophyll, it excites electrons which are then transported through an electron transport chain. This process creates an electrochemical gradient which powers ATP synthase to produce ATP.
So in summary, chloroplasts are the organelles where light energy is transformed into chemical energy in the form of ATP via photosynthesis. The thylakoid membranes and chlorophyll pigments absorb sunlight and facilitate this energy conversion through a series of reactions. This ATP can then be used by the plant cell as its source of chemical energy.
Conclusion
In summary, chemical energy stored in the bonds of glucose and other organic molecules is transformed into electrical energy within cells through a series of reactions and processes. The glycolysis pathway breaks glucose down into pyruvate, generating some ATP. Pyruvate enters the Krebs cycle within the matrix of the mitochondria, where more ATP is produced through substrate-level phosphorylation. As these reactions occur, electrons are stripped off the substrates and pass through the electron transport chain on the inner mitochondrial membrane. This creates an electrochemical proton gradient that drives ATP synthase to make more ATP via oxidative phosphorylation. The proton flow powers ATP synthase to phosphorylate ADP into ATP – transforming the electrochemical gradient into chemical energy stored in ATP. A similar process occurs in chloroplasts during photosynthesis, where light energy is used to build up the proton gradient. So in summary, step-wise breakdown of glucose and flow of electrons through redox reactions allows the cell to harness the energy in chemical bonds to generate an electrical gradient which is then used to power ATP production.