How Many Times Can Energy Change Forms?
Energy is the ability to do work or produce heat. There are many different forms of energy such as mechanical, thermal, chemical, nuclear and electromagnetic. The law of conservation of energy states that energy can neither be created nor destroyed, only transformed from one form to another. In an isolated or closed system, the total amount of energy remains constant, though it changes forms through energy transformations.
Some common forms of energy include:
- Mechanical – the energy of motion, eg kinetic or potential energy
- Thermal – heat energy
- Chemical – energy stored in the bonds of atoms and molecules
- Nuclear – energy stored in the nucleus of an atom; released through fission, fusion or radioactive decay
- Electromagnetic – energy of electromagnetic radiation like light or radio waves
The law of conservation of energy is one of the basic laws of physics. It states that within a closed system, the total amount of energy remains constant. Energy can only change form through energy transformations. For example, chemical energy in gasoline can be converted into kinetic energy to move a car. But the total amount of energy before and after remains the same.
Forms of Energy
The main forms of energy include:
-
Thermal Energy – The energy of moving molecules. It is the internal energy in substances and related to temperature.
-
Radiant Energy – Energy that travels in the form of electromagnetic waves. Examples are visible light, ultraviolet light, infrared waves, microwaves, radio waves, and X-rays.
-
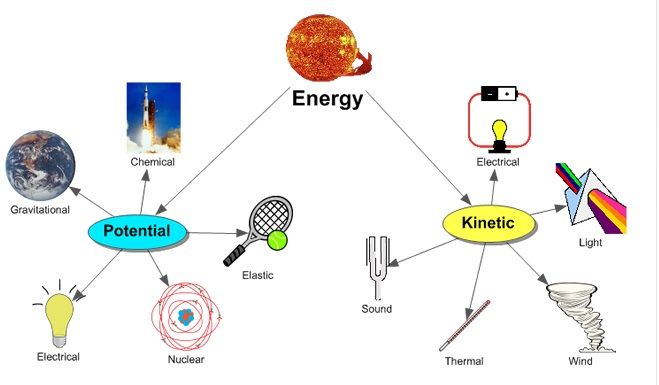
Kinetic Energy – The energy of motion. Moving objects and particles have kinetic energy.
-
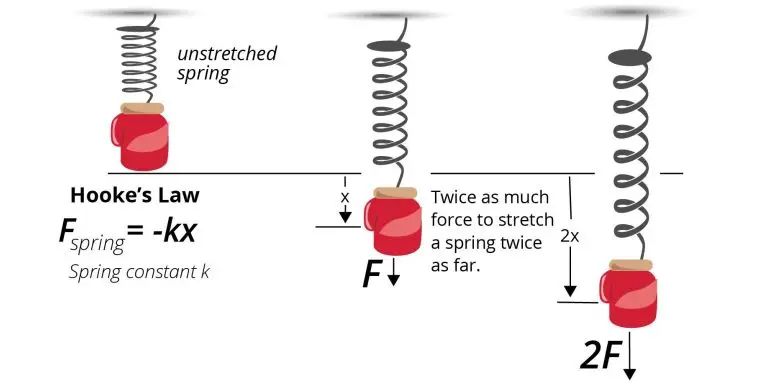
Potential Energy – Stored energy that an object has due to its position or chemical composition. Examples are gravitational potential energy from height, elastic potential energy from deformation, and chemical potential energy from chemical bonds.
-
Electrical Energy – Energy from the movement of charged particles like electrons. Electrical energy is used to power devices.
-
Chemical Energy – Energy stored in the bonds between atoms and molecules. Chemical energy can be released in chemical reactions.
-
Nuclear Energy – Energy stored in the nucleus of an atom, released in nuclear reactions. Nuclear energy comes from radioactive materials like uranium.
Energy Transformations
Energy can change from one form to another through various processes. Here are some common examples of energy transformations:
Chemical to Light Energy
Chemical energy stored in materials like wood, oil, or batteries can be converted into light energy through combustion, electricity, or chemical reactions. For example, burning wood releases chemical energy as heat and light.
Electrical to Mechanical Energy
Electricity can be converted into motion or mechanical energy through electric motors. This allows electrical energy to perform work like powering electric vehicles, kitchen appliances, power tools, and more.
Radiant to Thermal Energy
Radiant energy from the sun can be absorbed by objects on Earth as thermal energy or heat. The warmth from sunlight is an example of radiant energy transforming into thermal energy.
Nuclear to Electrical Energy
Nuclear power plants convert nuclear energy released from the splitting of uranium atoms into electrical energy that can power homes and businesses.
Energy transformations occur continuously all around us. The ability of energy to change forms allows it to be harnessed in useful ways through technology to meet human needs.
Law of Conservation of Energy
The law of conservation of energy states that energy can neither be created nor destroyed, it can only be transformed from one form to another. This means the total amount of energy in an isolated system always remains constant.
For example, when a book falls off a table, the potential energy that was stored due to its position on the table transforms into kinetic energy as the book gains speed while falling. The initial amount of potential energy is equal to the final amount of kinetic energy gained by the book (ignoring small losses due to air resistance and friction).
This law applies to all isolated or closed systems, where no external energy can enter or escape. However, the law does not hold true for open systems, where energy can cross the system boundary. For instance, living organisms are open systems that exchange energy with their surroundings in the form of food, heat, work, etc. The law of conservation of energy helps describe and predict energy transformations within closed systems.
Closed vs Open Systems
When it comes to energy transformations, there is an important distinction between closed and open systems. A closed system is isolated from its surroundings and does not exchange energy or matter outside of the system boundaries. For example, a bouncing ball on a table is a closed system – the ball exchanges energy between kinetic and potential within the system, but no energy crosses the system boundary. In contrast, an open system can exchange energy and matter with its surroundings. For example, a car engine takes in fuel and oxygen, transforms chemical energy into heat and mechanical energy, and releases exhaust – so it is exchanging matter and energy with its surroundings.
This distinction between closed and open systems is crucial for energy transformations. In a closed system, the total quantity of energy remains fixed according to the law of conservation of energy. Energy can transform between different forms, but the net quantity is always conserved. However, in an open system energy can be gained or lost via transfers across the system boundary. While energy must still be conserved globally, the quantity within an open system can change. This allows many more possibilities for energy transformations in open systems found commonly in nature and technology.
Understanding the behavior of closed versus open systems is key to analyzing energy transformations. Closed systems place limits on the potential changes, while open systems allow energy to be harnessed more flexibly from outside sources.
Real World Examples
Energy transformations happen continuously all around us. Here are some common real world examples:
Generators
Generators convert mechanical energy into electrical energy. In hydroelectric generators, the kinetic energy of moving water spins a turbine which spins coils of wire inside a magnetic field, generating electricity.
Batteries
Batteries convert chemical energy into electrical energy. Chemical reactions inside the battery cause electrons to flow through an external circuit, powering electrical devices.
Food chains
Animals get their energy from food, converting chemical energy from plants or other animals into kinetic energy to move around. At each step of the food chain, some energy is lost as heat during digestion and metabolism.
These are just a few everyday examples of the many different types of energy transformations constantly taking place around us.
Energy Losses
When energy transforms from one form to another, some energy is often lost in the process. This lost energy is no longer available to do useful work. There are several ways energy can be lost during energy transformations:
-
Friction – Movement causes friction, which converts useful kinetic energy into heat. This heat is often wasted into the environment.
-
Sound – Useful energy transforming into sound waves is often considered lost or wasted energy.
-
Imperfect design – No machine is 100% efficient. Design limitations cause wasted energy like heat, friction, resistance, etc.
-
Dissipation – Some energy forms naturally dissipate over time when transformed, like heat dissipating into the air.
-
Inaccessible energy – Energy can become practically inaccessible even though it still exists theoretically, like heat energy dispersed into the ocean.
Minimizing energy losses is an important engineering goal when designing efficient energy transformation systems. But some loss is inevitable. Most machines only convert 25-60% of input energy into useful output energy.
Unlimited Transformations
In theory, energy can change from one form to another an unlimited number of times. This is because energy is never created or destroyed according to the law of conservation of energy. Energy is simply converted between different forms.
For example, chemical energy in food can be converted to kinetic energy as a person uses that energy to ride a bicycle. The motion of the bicycle can be used to spin a generator which converts the kinetic energy into electrical energy. That electricity could then power a light bulb, converting the electricity into light and thermal energy. The cycle could keep going, transforming the thermal energy into another form of energy.
There is no theoretical limit to how many times energy can change forms. As long as the system is efficient enough, the energy could keep transferring between different states indefinitely. Each change of form is called an energy transformation. The number of potential energy transformations is essentially limitless.
Practical Limits
While in theory energy can transform an unlimited number of times according to the law of conservation of energy, in practice there are limits. This is because real-world systems experience energy losses during energy transformations. Some examples of energy losses include:
- Friction converting useful mechanical energy into heat
- Heat transfer from hot to cold objects
- Electrical resistance converting electricity into heat
- Sound and light waves dissipating into the surroundings
These losses build up over multiple energy transformations, decreasing the total amount of useful energy available to do work. For example, when burning gasoline in a car engine, some energy is lost as heat in the engine, some energy goes into sound from the engine noise, and additional energy is lost through friction between the tires and road. After multiple energy transformations, the original chemical potential energy in the gasoline is diminished, putting a practical limit on the number of possible transformations.
While energy can theoretically transform indefinitely, real systems experience degradation over time. Devices require periodic maintenance and refueling to counteract these losses. So in practice, the number of possible energy transformations has limits based on conversion inefficiencies and energy dissipation.
Conclusion
In summary, energy is able to change between different forms, such as from chemical potential energy to kinetic energy. As discussed earlier, the law of conservation of energy states that energy cannot be created or destroyed, only transformed from one form to another. So theoretically, there is no limit to how many times energy can change forms.
However, in practical real-world systems, some energy is always lost or wasted, usually in the form of heat. For example, when burning gasoline in a car engine, not all of the chemical energy is converted into kinetic energy to move the car. Some energy is lost due to friction, sound, heat dissipation, etc. Although the total amount of energy is conserved, these losses limit the amount of useful energy available.
Therefore, while energy can theoretically change forms an unlimited number of times, there are always some practical inefficiencies. With each energy transformation, a percentage of energy becomes unavailable for further useful work. So in real systems, the number of energy transformations is constrained by these unavoidable energy losses.