How Is Thermal Transferred?
Introduction
Thermal energy transfer refers to the movement of heat from one object or system to another as a result of their temperature difference. There are three main mechanisms by which thermal energy can be transferred between objects or systems:
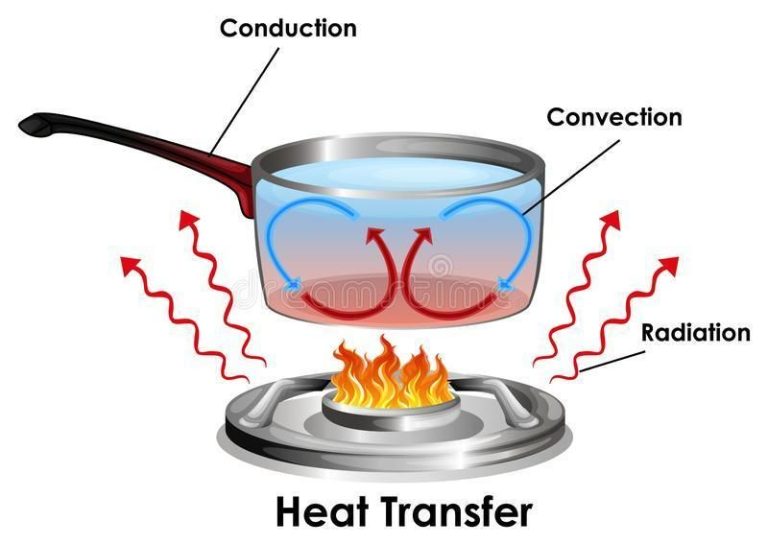
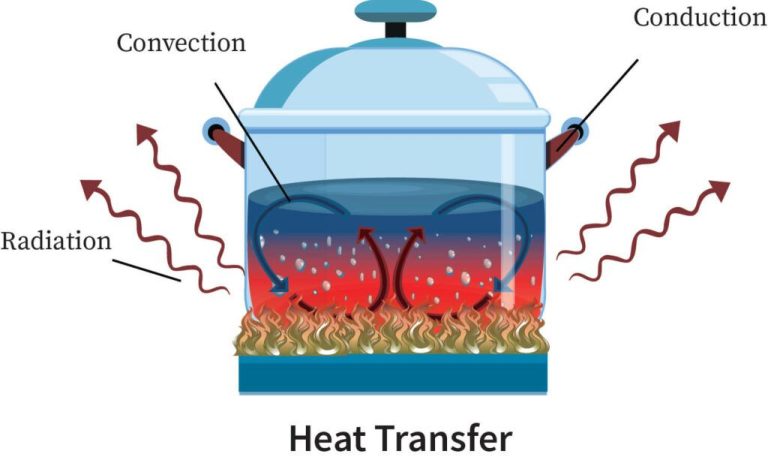
– Conduction: The transfer of thermal energy between substances in direct physical contact. Heat moves from warmer to cooler parts of an object or between two objects in contact.
– Convection: The transfer of thermal energy by the bulk movement of fluids (liquids or gases). Hotter fluids rise due to lower density, while cooler fluids sink, causing circulation.
– Radiation: The transfer of energy by electromagnetic waves directly from one surface to another without heating the space in between. All objects emit thermal radiation related to their temperature.
This article will provide an overview of these three main methods of thermal energy transfer, explaining the physics behind each one, comparing their relative effectiveness, and providing real-world examples of how they impact our lives.
Conduction
Conduction is the transfer of heat through a material by direct contact. It occurs when atoms and molecules vibrate against each other, transferring kinetic energy. Heat moves from the warmer region to the cooler region until both reach the same temperature.
A common example of conduction is heating a metal pan on a stovetop. The heat from the stovetop burner is conducted through the metal molecules in the pan. The metal molecules vibrate rapidly next to the heat source, transferring the energy to neighboring metal molecules farther away. This continues until the entire pan reaches a uniform hot temperature.
Metals like copper and aluminum are good conductors as their atoms readily share vibrational energy. Insulators like wood or plastic do not conduct heat well because their molecular structures inhibit energy transfer.
Conduction can only occur within or between solids that are touching. It does not transfer heat through air or liquids. Conduction is most effective over short distances and works better in some materials than others.
Convection
Convection is another mechanism for thermal transfer. It involves the movement of heated fluids and gases. For example, when a liquid or gas is heated, it becomes less dense and rises upward. This circulation and mixing allows heat to spread throughout the material.
A common example of convection is hot air balloons. The burner heats the air inside the balloon, making it expand and become lighter than the surrounding air. This allows the balloon to rise. The cooler air then moves in underneath the balloon, causing circulation.
Convection currents are another everyday example. When water is heated in a pot, the hotter water at the bottom rises while the cooler water sinks. This motion allows the heat to spread evenly throughout the water, rather than being concentrated at the bottom.
The heating of rooms also relies heavily on convection. Hot air near heating vents or radiators rises upward, heating the rest of the room as it circulates.
Convection plays a major role in global air and ocean currents. It allows heat to circulate around the planet through fluid dynamics.
Radiation
Thermal radiation is the transfer of heat via electromagnetic waves. All objects emit thermal radiation related to their temperature. Hotter objects emit more thermal radiation than colder objects. This is how the sun transfers heat to the earth – the sun emits high levels of thermal radiation which travels through space in the form of light and infrared waves until being absorbed by the earth’s surface, heating it up.
Common examples of thermal radiation on earth include:
- The heat that you feel from a fire or heating element. The waves travel through the air and are absorbed by your skin.
- Infrared cameras can visually detect thermal radiation being emitted by objects, allowing you to see variations in temperature. Warm objects like people and animals will stand out from cooler backgrounds.
- Greenhouse gases in the atmosphere absorb thermal radiation emitted from the earth’s surface. This absorption leads to atmospheric heating that contributes to climate change.
Thermal radiation does not require a medium to transfer heat. It can travel through empty space, unlike conduction and convection which rely on direct contact or fluid movement. Thermal radiation is the only way for the sun’s heat to reach Earth across the vacuum of space.
Comparing Methods
The three main methods of thermal energy transfer—conduction, convection, and radiation—work in different ways to move heat from one place to another. Here is a comparison of how they function:
Conduction relies on direct contact between materials, allowing thermal energy to transfer between atoms and molecules. It can only occur in solids, liquids, and dense gases. Conduction works best between materials with similar structures that are tightly bonded.
Convection requires a fluid (liquid or gas) to transfer heat by the bulk movement or currents that develop in that fluid when it is heated. Hot, less dense material rises while cooler, denser material sinks, creating circulation patterns that carry heat.
Radiation transfers thermal energy directly through space via electromagnetic waves and does not rely on direct contact between materials or fluid movement. It can travel through solids or empty space. All objects above absolute zero emit thermal radiation.
While conduction and convection rely on matter to transfer heat, radiation can transfer heat across a vacuum. Conduction transfers heat slower than convection and radiation. Convection will only occur in fluids, while conduction requires direct contact between materials. Radiation does not rely on contact or fluid movement at all.
Real World Examples
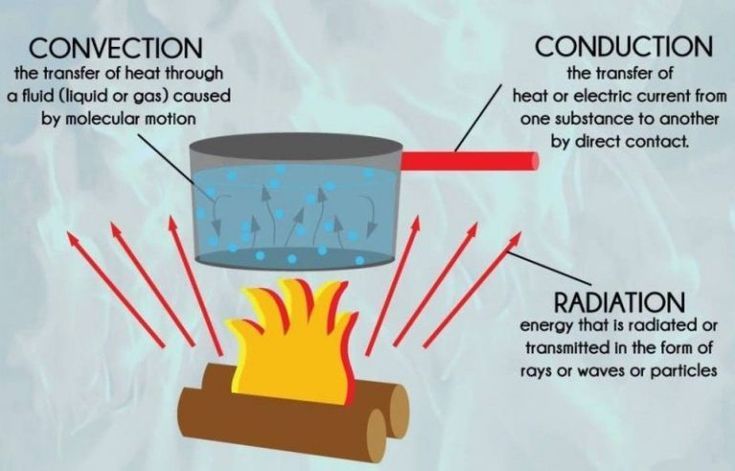
Heat is constantly being transferred in the world around us through conduction, convection, and radiation. Here are some everyday examples of each:
Conduction:
- Touching a hot stove – the metal pan conducts heat to your hand.
- Wrapping your cold hands around a warm mug of coffee – the heat from the liquid conducts through the mug to your hands.
- Wearing a metal cooking pot holder while taking food out of the oven – the metal quickly transfers the oven’s heat to your hand, so an insulated holder is better.
Convection:
- Hot air rising from a radiator or vent, heating the room.
- A pot of water boiling on the stove as heated liquid at bottom rises while cooler liquid sinks.
- Warm air currents rising in the atmosphere, creating weather patterns.
Radiation:
- Feeling the warmth of the sun’s rays shining down.
- Using a microwave oven that cooks food using radiant heat.
- Turning on an infrared heater that directly heats objects in a room.
As you can see, heat transfer occurs all around us through various methods. Understanding these principles allows us to harness conduction, convection, and radiation effectively in many applications.
Applications
Thermal energy transfer has many important applications in technology and engineering. Here are some key examples:
Heat sinks and heat exchangers: These devices utilize conductive and convective heat transfer to move thermal energy away from sensitive components like computer processors. Fins help increase surface area for more effective convection cooling.
HVAC systems: Heating, ventilation and air conditioning rely on conduction, convection and radiation to heat, cool and ventilate buildings. Convection moves heat through ductwork while radiators and vents use radiation and natural convection for space heating.
Solar thermal energy: Sunlight is captured as thermal radiation to heat water and spaces. Solar thermal collectors maximize absorption of radiant heat.
Cooking appliances: Stovetops, ovens and grills use conduction to transfer heat into pots and food. Convection moves hot air and steam for even heating and browning.
Power generation: Many power plants generate electricity via heat engines like turbines. Heat is added to a working fluid that expands to drive turbines and generate power.
Impacts
Thermal transfer can have significant impacts on the environment. The main way it affects the environment is through climate change. The greenhouse effect, which is caused by gases like carbon dioxide trapping heat, is an example of radiative thermal transfer. This process leads to global warming as more heat is retained on Earth. Conduction and convection also play roles in moving heat around the planet and impacting weather patterns and ecosystems.
Another major environmental impact is thermal pollution. This occurs when power plants and industrial processes release waste heat into waterways and the atmosphere. The added heat can disrupt ecosystems that are adapted to certain temperature ranges. Thermal pollution can also decrease water quality and lead to depleted oxygen levels that harm aquatic life.
In terms of solutions, improving insulation, transitioning to renewable energy sources like solar and wind, and upgrading to more efficient heat transfer systems can help reduce thermal transfer’s environmental footprint. Better understanding heat flow through research helps scientists identify ways to mitigate climate change impacts and thermal pollution. Overall, responsible thermal management is key to reducing negative environmental effects.
Future Research
Thermal transfer is an active area of research with many exciting new developments on the horizon. Scientists are exploring innovative ways to manipulate and control heat flow for a wide range of applications.
One promising area is the development of novel engineered materials with enhanced thermal properties. Researchers are designing metamaterials, nanostructures, and other exotic substances to conduct, convect, or radiate heat in precise ways. These could enable breakthroughs in thermal management for electronics, buildings, and industrial processes.
Advances in nanotechnology and microfluidics are opening up new possibilities for manipulating heat at the smallest scales. Tiny pumps, valves, and switches embedded in materials could allow dynamic control over heat flow. This kind of active thermal management could revolutionize cooling for computer chips and other devices.
Mathematical modeling and simulation of thermal transfer processes are also accelerating. High-fidelity multiphysics simulations allow researchers to gain insights and optimize designs virtually. Machine learning techniques are being applied to discover unconventional approaches and materials for managing heat.
While foundational thermal transfer principles remain unchanged, researchers continue expanding the horizons of how we can control heat for our benefit. Exciting innovations that enhance efficiency, reduce waste, and enable novel applications can be expected in the future.
Conclusion
There are three main methods of thermal transfer: conduction, convection, and radiation. Each method has its own distinct processes for how heat energy is transferred from one location to another.
Conduction involves direct contact between materials, with energy transferred through collisions between atoms and molecules. Metals are good conductors. Convection relies on the movement of fluids like air or water to transfer heat. As the fluid circulates, it brings energy along with it from one location to another. Radiation works through electromagnetic waves that can travel long distances without relying on direct contact or fluid movement to propagate heat.
These three methods work in tandem in real world systems, from heating and cooling systems to weather patterns and beyond. Understanding how thermal energy is transferred is key to properly designing and optimizing these systems.