How Is Thermal Energy Transferred In Solids?
Thermal energy transfer refers to the movement of heat from one object or system to another as a result of temperature differences. There are three main methods of thermal energy transfer: conduction, convection, and radiation.
Conduction is the transfer of heat between objects that are in direct contact with each other, such as a pot on a hot stove. Metals tend to be good conductors while nonmetals like wood and plastic are poor conductors. Convection is the movement of heat via the circulation or flow of liquids and gases. As they move, they carry thermal energy from one place to another. Radiation does not rely on direct contact or fluid flow. Instead, it works by electromagnetic waves that can travel through empty space.
In this article, we will explore in detail how thermal energy is transferred through solids via the methods of conduction, convection, and radiation. Examples from everyday life will illustrate the principles, and we’ll learn about methods of insulation to reduce or prevent heat transfer.
Conduction
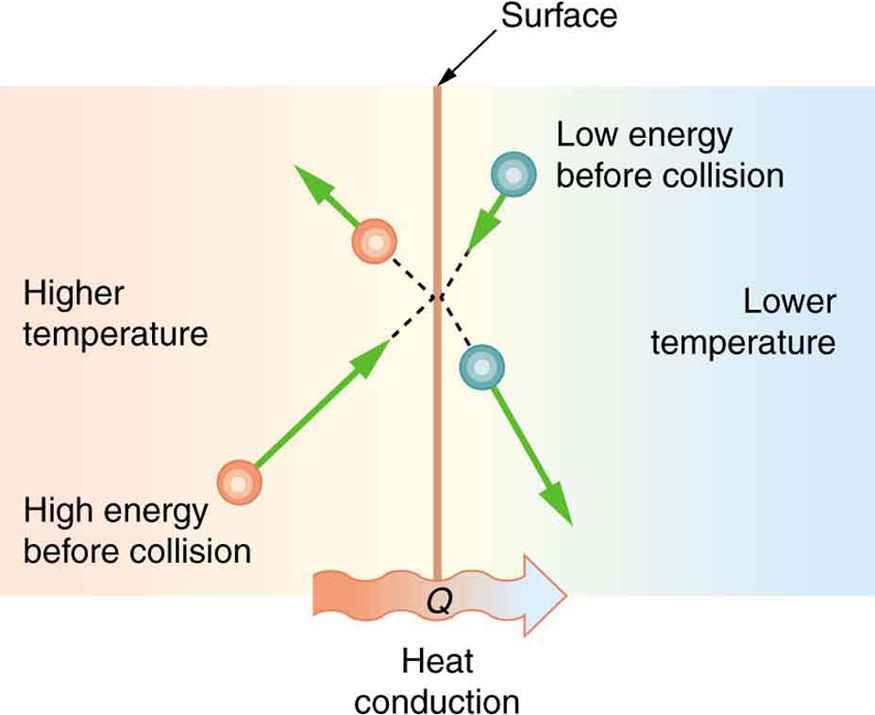
Conduction is the transfer of thermal energy between molecules in a solid material. It occurs when atoms vibrate and transfer their kinetic energy to neighboring atoms through direct contact. The warmer atoms vibrate more rapidly, colliding with nearby cooler atoms and increasing their vibration.
At the molecular level, heat transfers from the hotter end to the colder end of an object. The vibrating atoms essentially “push” their energy to adjacent atoms. The rate of conductive heat transfer depends on the temperature gradient and the thermal conductivity of the material.
Materials like metals have free electrons that can transfer thermal energy rapidly through the solid. This makes metals good conductors of heat. Nonmetals like wood, plastic and rubber have fewer free electrons, so they conduct heat more slowly.
Other factors that affect conduction rate include the cross-sectional area of the material (more area allows faster transfer) and the distance the heat must travel (shorter distance is faster). Insulators slow conduction by trapping air pockets that resist heat transfer.
Metals vs nonmetals
Metals are good conductors of thermal energy, whereas nonmetals are generally poor conductors. This difference arises from the molecular structure of metals versus nonmetals.
In metals, atoms readily give up their outermost electrons, forming a sea of free electrons that can transport thermal energy rapidly through the material. The positive metal ions form a fixed crystal lattice that the free electrons can flow through.
In contrast, nonmetals have tightly bound electrons that are not free to roam throughout the material. This makes it much more difficult for thermal energy to be transferred from atom to atom in nonmetals.
Convection
Convection is a process of heat transfer that occurs in fluids and gases. Since solids do not flow, convection does not occur in solids. Convection involves the circulation or movement of heated fluid away from the heat source and the simultaneous replacement with cooler fluid. The cooler fluid is then heated and the cycle continues. This process works because hot fluids expand, become less dense and rise while cold fluids become denser and sink. The energy is transferred through the motion and mixing of hot and cold fluids.
Convection can occur naturally or be induced through forced circulation. An example of natural convection is the currents that form in water that is being heated on a stove. The heated water at the bottom of the pot expands, becomes less dense, and rises while the cooler water sinks to replace it. This creates circulation currents. Forced convection occurs when mechanical devices like fans or pumps induce fluid motion, such as the heating and cooling systems in homes.
Radiation
Thermal radiation is the transfer of energy through electromagnetic waves. Radiation can occur between any two objects that have a temperature above absolute zero. Unlike conduction and convection, radiation does not require any medium for energy transfer.
All objects emit thermal radiation related to their temperature. This radiation is in the form of infrared rays that travel at the speed of light. The hotter the object, the shorter the wavelength and higher frequency of the infrared rays emitted. When these rays strike another object, they are absorbed and converted to thermal energy, heating that object.
Radiation allows heat transfer over large distances, even across vacuums of space. This is how the heat of the sun reaches Earth. Thermal radiation does not require interactions between molecules like conduction and convection. It occurs between any objects with a temperature differential across empty space.
Real-world examples
Thermal energy is constantly being transferred in the objects around us through conduction, convection, and radiation. Here are some everyday examples:
Conduction:
- Heat traveling through a frying pan as you cook
- Warmth from your body being transferred to a metal chair when you sit down
- Heat moving through clothing from your skin to the outer layers
Convection:
- Hot air rising from a radiator or heat vent
- Heat circulating in an oven or pot on the stove
- Warm air currents rising in a room with a fireplace
Radiation:
- Heat from the sun warming your skin
- Heat radiating from hot coals in a campfire
- Warmth radiating from a heated floor or coiled electric stove
We experience these types of heat transfer daily through our heating systems, clothing, cooking appliances, and more. Understanding how thermal energy moves allows us to design better insulation for homes, protect ourselves from extreme temperatures, and efficiently harness energy from heat sources.
Methods of Insulation
Insulation is used to slow the transfer of heat energy between objects. There are several materials and methods used for insulation in buildings, appliances, and clothing.
Common insulation materials include fiberglass, mineral wool, cellulose, polystyrene foam, and polyurethane foam. These are used in walls, ceilings, floors, and attics in homes and buildings to reduce heat transfer and lower energy costs. Thick layers or batts of insulation are placed in the spaces between wall studs, roof rafters, and floor joists.
Appliances like ovens and refrigerators have insulation built into their walls to prevent heat transfer and allow them to maintain different temperatures from the surrounding environment. This keeps food hot or cold. Insulated packaging is also used to ship temperature sensitive items.
Clothing and winter gear uses insulation to retain body heat. Down feathers, fleece, and wool provide insulation by trapping air pockets. Layers of clothing with dead air space between them can also insulate the body.
Proper insulation is an important consideration in energy efficiency, appliance functionality, and comfort across many aspects of everyday life.
Thermal Energy Transfer in Earth
The Earth’s interior contains a tremendous amount of thermal energy that is constantly being transferred and circulated. There are three primary mechanisms for this transfer: conduction, convection, and radiation.
Conduction occurs in the Earth’s crust and mantle, which are solid rock. Heat travels through these layers slowly as warmer molecules transfer thermal energy to adjacent cooler molecules. The outer core is also solid and conducts heat from the inner core outward.
Convection occurs in the magma (molten rock) of the mantle and outer core. As magma heats up, it becomes less dense and rises while cooler magma sinks. This circulation allows heat to be transported from deep within the Earth towards the surface.
The Earth’s primary internal heat source is residual heat from planetary accretion during formation as well as decay of radioactive elements. The inner core is the hottest region at roughly 5,700°C due to pressure and residual heat. This heat slowly conducts outwards through the core and mantle, driving mantle convection.
Applications
Thermal energy transfer has many practical uses and applications in our everyday lives and in various industries.
In electronics and computing, understanding thermal conduction helps manage heat dissipation from devices like computer processors and mobile phone chips. Heat sinks and cooling fans rely on conduction and convection to prevent overheating.
In manufacturing and metalworking, knowledge of thermal properties allows control of temperatures during processes like welding, casting, heat treating, and more. Induction and laser heating apply principles of radiation and conduction.
In cooking, conduction heats pans on stoves as burners transfer thermal energy. Ovens rely heavily on thermal radiation and convection for baking and roasting. Microwaves use electromagnetic radiation to excite water molecules and generate heat.
Building insulation blocks conductive and convective heat transfer to conserve energy. Radiant barriers also help reduce cooling/heating loads. Windows can be designed to optimize radiation from sunlight.
Understanding conduction allows proper use of heat pipes and thermosiphons to transfer thermal energy in applications like electronics cooling, solar water heating, and more.
Summary
In summary, there are three main methods of thermal energy transfer in solids: conduction, convection, and radiation. Conduction involves direct transfer of heat between molecules of a solid material. Metals tend to be good thermal conductors, while nonmetals like wood are poor conductors. Convection relies on the movement of heated fluids like air or water to transfer thermal energy. Radiation works through electromagnetic waves emitted by hot objects. Understanding how thermal energy is transferred in solids helps explain many everyday phenomena, from cooking on a stove to insulating our homes. It also has important applications in fields like engineering and weather science.
Being familiar with the principles of conduction, convection, and radiation provides vital insight into how thermal energy moves through solid materials. This knowledge enables the design of effective insulation, heating, and cooling systems. It also aids in predicting and responding to weather patterns that involve thermal energy flow. With a grasp of these basic methods of heat transfer, one can better comprehend the thermal behavior of objects and systems in everyday life.