How Is Light Emitted Or Produced?
Light is a fundamental part of the natural world, and understanding how it is produced and emitted is key to unlocking many scientific principles. In this article, we will provide an overview of what light is and the various ways it can be emitted or produced. We will cover light emission from stars through processes like nuclear fusion, as well as artificial light production through incandescence, lasers, LEDs, and more. Key concepts such as spectra, photon excitation, and radiation will help explain what affects light emission across different contexts. By the end, you will have a broad understanding of how this crucial form of electromagnetic radiation is generated by natural and artificial means.
What is Light?
Light is a form of electromagnetic radiation that is visible to the human eye. Electromagnetic radiation consists of oscillating electric and magnetic fields that propagate or travel through space in the form of waves. The wavelength and frequency determine the properties of electromagnetic radiation.
Visible light is the portion of the electromagnetic spectrum that is visible to the human eye. The wavelength of visible light ranges from about 380 to 740 nanometers. Beyond these wavelengths lie the ultraviolet (shorter wavelengths) and infrared (longer wavelengths) portions of the electromagnetic spectrum.
The frequency and wavelength are inversely related – as the frequency increases, the wavelength decreases. The frequency of visible light ranges from 430 to 790 terahertz. As the frequency increases, the energy of the light also increases.
All forms of electromagnetic radiation travel at the same speed – the speed of light – which is about 3 x 10^8 meters per second in a vacuum. This is an important universal constant.
So in summary, light is a type of electromagnetic wave characterized by wavelengths visible to the human eye, with properties such as frequency and energy that depend on the wavelength.
Light Emission from Stars
Nuclear fusion in a star’s core produces intense heat and outward pressure, causing the star to glow brightly. Within the core, hydrogen atoms fuse into helium, releasing energy in the form of gamma rays. As these gamma rays work their way outward from the dense core, they continuously collide with and energize atoms in the star’s interior layers.
The energized atoms emit photons as the electrons shift between different orbital states. The photons interact with more atoms on their way out, gradually shifting to lower frequencies as they approach the star’s surface. By the time the photons reach the outer layers and radiate into space as visible light, they have been “thermalized” into a range of wavelengths emitting the star’s characteristic color.
Hot blue stars peak in the blue and ultraviolet end of the spectrum, while relatively cool red stars emit mostly red and infrared light. The color of a star depends on its surface temperature and thus indicates its stage of stellar evolution. Younger, hotter stars appear bluish-white while older stars trend red as they exhaust their core fuel over billions of years.
Incandescence
Incandescence is a form of light emission that involves heating a material until it starts to glow and emit light. One of the most common examples of incandescence is seen in an incandescent light bulb. In an incandescent light bulb, a very thin metal wire called a filament is enclosed in a glass bulb. When current passes through the filament, it heats up to very high temperatures, causing the filament to glow and produce visible light. This glow is known as incandescence.
The hotter the filament gets, the brighter it glows. Incandescent light bulbs work by precisely controlling the temperature and therefore the glow of the filament inside. The same principle of incandescence applies to metals that are heated until they are red, orange, or yellow hot. This reddish glow is the metal incandescing at that temperature. Incandescence produces light due to the heat and has nothing to do with luminescence or fluorescence. Any material that can be heated to a high enough temperature will start to visibly glow and emit light through incandescence.
Luminescence
Luminescence refers to the emission of light from substances that are not hot like incandescent objects. There are several types of luminescence including chemiluminescence, bioluminescence, fluorescence, and phosphorescence.
Chemiluminescence involves a chemical reaction that produces light as one of its products. A common example is the glow sticks that are often used at concerts and parties. These contain chemicals that when mixed together, undergo a reaction that generates light.
Bioluminescence is light produced by living organisms through specialized biochemical reactions. Some examples are fireflies, glowworms, certain bacteria, and deep sea creatures that use bioluminescence for purposes like attracting prey, camouflage, or mating signals.
Fluorescence and phosphorescence are types of photoluminescence, where substances absorb light energy and re-emit it. The main difference is that fluorescent materials re-emit light immediately when excitation stops, while phosphorescent ones have a delayed emission that can persist for minutes or hours after the initial excitation.
Lasers
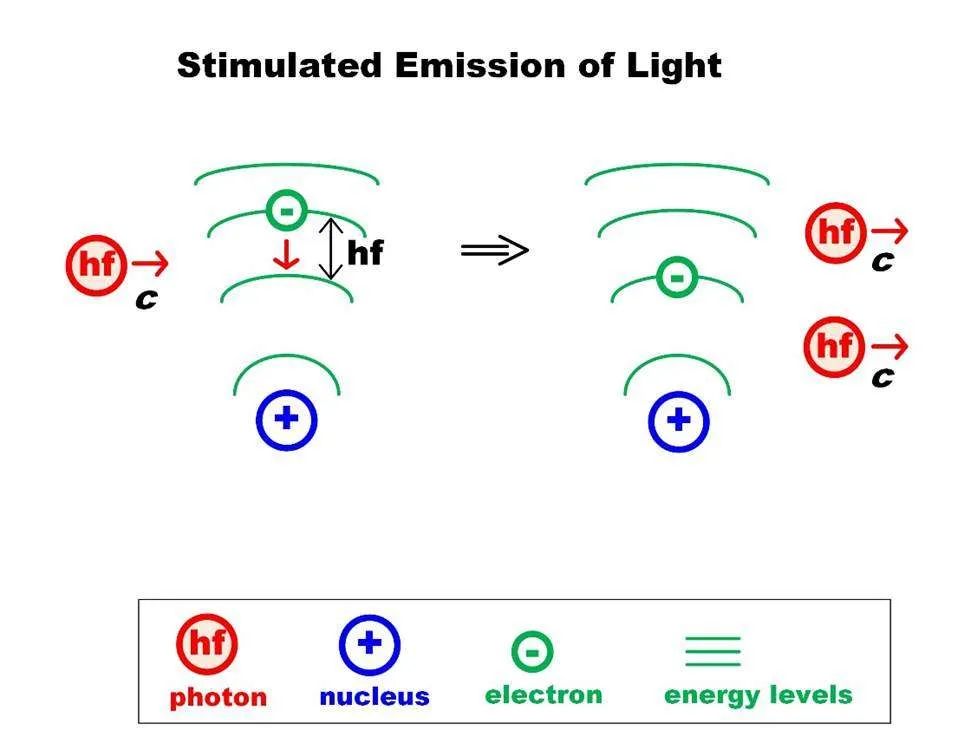
Lasers produce light through a process called stimulated emission. Inside a laser device, there is a material called the lasing medium which can be a solid, liquid or gas. This lasing medium contains atoms that can be stimulated to emit photons or particles of light.
To stimulate these atoms, the lasing medium is pumped with energy, usually from a flashlamp or electrical discharge. This pumping excites the atoms to higher energy states. The excited atoms are then stimulated to emit photons of the same wavelength and phase, creating a cascade effect resulting in an intense, focused beam of coherent light. This stimulated emission only occurs within an optical cavity containing mirrors that reflect the photons back and forth, amplifying the beam.
The intense coherence and high beam quality make lasers useful for many applications including laser cutting and welding in manufacturing, laser surgery in medicine, laser printing, barcode scanning, laser pointers, and fiber optic communications that transfer data via laser light pulses through fiber cables. Lasers have become an integral technology enabling advanced applications involving intense beams of controlled, monochromatic light.
LEDs
LEDs, which stands for Light Emitting Diodes, create light through a phenomenon called electroluminescence which occurs when electrons move through a semiconductor material. When a voltage is applied across the semiconductor junction in an LED, electrons are able to move into an area with excess holes. When the electrons recombine with the holes, energy is released in the form of photons or light particles.
The color or wavelength of the emitted light depends on the type of semiconductor material used. For example, Gallium Arsenide Phosphide emits red light, Gallium Phosphide emits green light, and Indium Gallium Nitride can produce blue and ultraviolet light. Combining different semiconductor materials allows LEDs to emit various colors and wavelengths.
LEDs have many advantages over traditional incandescent light bulbs. They are energy efficient, converting over 80% of energy into light rather than heat. LEDs also have long lifespans, lasting anywhere from 25,000 to 100,000 hours. They can be small in size while producing bright, focused light. LED technology also enables advanced applications like full color video displays. With their durability, efficiency and versatility, LEDs are becoming increasingly used for lighting, displays, and indicators across consumer electronics, automotive, medical, and industrial sectors.
Other Light Sources
There are a few other interesting light sources that are worth mentioning briefly:
Cathode Ray Tubes (CRTs) – CRTs were commonly used in older television sets and computer monitors. They work by firing electrons at a phosphor coating on the inside of the glass screen. When the electrons strike the phosphor, it emits visible light.
Plasma Displays – These displays, sometimes called plasma screens, contain neon and xenon gases between two glass panels. Electrodes excite the gases, causing them to emit ultraviolet light. This UV light then strikes phosphors on the screen, causing them to glow different colors.
Fluorescent Lamps – Fluorescent lights contain mercury vapor that emits UV light when ionized. The UV light causes a phosphor coating inside the bulb to glow and emit visible light.
Neon Lights – These lights contain neon or other gases in glass tubes. Electricity excites the gas atoms, causing them to emit colored light as they return to lower energy states.
While not as common today, these older technologies paved the way for many of our modern light sources that we use and take for granted.
What Affects Light Emission?
The properties of light, including wavelength/color, intensity, and directionality, can be influenced by the underlying mechanism of light emission.
Wavelength and color are determined by the energy level changes undergone by electrons in atoms, molecules, or other particles. When an electron transitions between different energy levels in an atom or molecule, a photon is emitted with energy that matches the precise energy difference between those quantum levels. Since photon energy is inversely related to wavelength, smaller energy changes result in longer wavelength photons (like infrared), while larger energy changes result in shorter wavelength, higher energy photons (like ultraviolet). The spectrum of wavelengths emitted is therefore highly dependent on the possible electron transitions in the light-emitting substance.
The intensity of emitted light depends on the number of electrons undergoing these energy transitions per unit time. More electrons making the jump means more photons emitted per second, resulting in a greater light intensity. Population inversion, where higher energy states have more electrons than lower energy states, greatly increases the intensity of light emission in laser cavities and LEDs.
The directionality of emitted light varies based on the mechanism. Thermal sources like incandescent bulbs emit light isotropically in all directions. Lasers produce a very narrow, focused beam of light in one direction. LEDs and fluorescent sources have directionality in between these two extremes.
Conclusion
As we’ve discussed, there are various natural and artificial processes that produce light through different mechanisms like incandescence, luminescence, and stimulated emission. Stars emit light through nuclear fusion reactions in their cores, while lamps and LEDs convert electricity into light. Lasers generate highly focused beams of light via stimulated emission of photons in special materials.
Light is a fundamental part of our universe that makes sight and life possible. Scientists continue researching light emission to discover new light sources like better LED bulbs or high-powered lasers. Understanding the science behind light allows us to harness it for important technologies that benefit society. While we’ve made great progress, the intricate ways matter can interact with light and produce photons remains an exciting area of study full of potential.