How Energy Is Transformed Into Electricity?
The field of electricity has a long history marked by fascinating discoveries and groundbreaking applications. It all began around 600 BC when Greek philosophers Thales of Miletus and Eratosthenes of Cyrene first observed that rubbed amber could attract and repel feathers. Fast forward to the 1600s when English physicist William Gilbert made the connection that this attraction was caused by electric force and coined the term “electric”. Progress accelerated in the 1700s as researchers like Benjamin Franklin, Luigi Galvani and Alessandro Volta pioneered the study of static, current and stored electricity.
The modern use of electricity really took off in the late 1800s with mass electrification spreading across many countries. In today’s world, electricity provides power for devices, heating, lighting and more in nearly every home, business and industry. Electrical energy itself refers to the movement of electrons which create charge. We rely on electricity so intimately in our daily lives. Learning more about how it is generated underscores its incredible importance.
Forms of Energy
Energy exists in many different forms that can be categorized into types. The main forms of energy relevant to electricity generation include:
Potential Energy
Potential energy is stored energy due to position or state. For example, a ball held at a height above the ground has potential energy due to gravity. When released, this potential energy transforms into kinetic energy as the ball falls. Sources like water reservoirs held behind dams have potential energy that can generate electricity.
Kinetic Energy
Kinetic energy is energy of motion. Moving objects and materials like wind, flowing water, and steam have kinetic energy that can be harnessed. For example, wind turbine blades capture the wind’s kinetic energy and transform it into rotational mechanical energy to drive an electrical generator.
Chemical Energy
Chemical energy is stored in the bonds between atoms and molecules and released in chemical reactions. Burning fossil fuels like coal, oil, and natural gas releases chemical energy that can heat water to produce steam to spin turbines. Batteries also store chemical energy and undergo reactions to produce electricity.
Nuclear Energy
Nuclear energy comes from binding energy stored within atomic nuclei and released in nuclear reactions. Nuclear fission of heavy elements like uranium produces heat that powers nuclear reactors and generates electricity. Nuclear fusion reactions of light nuclei also release energy but have yet to be harnessed for electricity generation.
Electrical Energy Basics
Electricity is a form of energy that arises from the flow of electric charge. There are several key concepts related to electrical energy:
Electric Charge: Electric charge is a fundamental property of matter that exists in positive and negative forms. It is carried by subatomic particles such as protons and electrons. The buildup of electric charge between two objects creates electric potential energy.
Electric Current: Electric current is the flow of electric charge. It is the rate at which charge passes through a given point. Current is measured in Amperes and represented by the symbol I.
Voltage: Voltage is the difference in electric potential between two points in a circuit. It is the “push” or “pressure” that causes electric current to flow. Voltage is measured in Volts and represented by the symbol V.
Electrical Power: Electrical power is the rate at which electrical energy is transferred by an electric circuit. It is the product of voltage and current. Power is measured in Watts and represented by the symbol P.
Electromagnetic Induction
Electromagnetic induction is the process by which electricity is generated from motion or magnetism. It was discovered in the 1830s by Michael Faraday, who found that moving a magnet near a coil of wire caused an electric current to flow in the wire. This phenomenon is described by Faraday’s law of induction.
Faraday’s law states that a changing magnetic field near a conductor, like a wire, will induce a voltage across the ends of the conductor. So by moving a magnet in and out of a coil of wire, the changing magnetic field generates a voltage that drives electrons through the wire, creating an electric current.
The same effect occurs if the coil of wire is moved through a stationary magnetic field. It’s the relative motion between the magnet and coil that causes the field to change and induce a voltage. Generators use this principle to convert kinetic energy into electrical energy. As the generator spins, it moves coils of wire through a magnetic field, inducing a voltage that creates a flow of electricity.
Electromagnetic induction forms the basis for how energy from motion and magnetism is transformed into electrical energy. Whether it’s a spinning turbine at a power plant, or the rotor inside an electric motor, electromagnetic induction enables the generation of electricity from motion.

Thermoelectric Effect
The thermoelectric effect refers to phenomena where temperature differences create electric voltage and vice versa. There are three main thermoelectric effects:
Seebeck Effect
The Seebeck effect describes how electricity can be generated using the difference in temperature between two dissimilar metals or semiconductors. If two wires made from different materials are joined at both ends to form a closed loop, and one junction is held at a higher temperature than the other, a voltage difference will develop between the two junctions. This voltage can drive an electric current around the circuit.
Peltier Effect
The Peltier effect is the reverse of the Seebeck effect. When an electric current flows through two dissimilar conductors, heat is absorbed at one junction and released at the other. This creates a temperature difference between the junctions. Peltier devices use this effect to create heat pumps and thermoelectric coolers.
Thermoelectric Generators
Thermoelectric generators utilize the Seebeck effect to convert heat directly into electricity. They are made of pairs of n-type and p-type semiconductor pellets connected electrically in series and thermally in parallel between two ceramic plates. Applying heat to one side and cooling the other creates voltage that can be used to power devices or charge batteries.
Photovoltaic Effect
The photovoltaic effect refers to the generation of voltage and electric current in a material upon exposure to light. It is the operating principle behind solar cells which convert sunlight directly into electricity.
When photons from sunlight hit the solar cell, they transfer their energy to the electrons in the solar cell material, causing them to break free from their atoms. The electrons can then flow through an external circuit, producing electricity. This process of electrons being emitted from a material due to light exposure is called the photoelectric effect.
Solar cells are made from semiconductor materials such as silicon, which are specially treated to form an electric field that forces the electrons to flow in one direction, producing direct current electricity. The most common solar cell material is crystalline silicon, but various other semiconductor materials are also used such as gallium arsenide, cadmium telluride, and copper indium gallium selenide.
Solar cells are connected together in panels or modules. Solar panels can vary in size depending on the application. Larger utility-scale solar power plants may have very large panels on ground mounts or tracker systems to maximize energy production. For residential rooftop systems, the solar panels are usually 1-2 square meters in size.
The photovoltaic effect allows solar panels to directly generate clean renewable electricity from sunlight. It is a key technology in enabling sustainable solar energy to displace fossil fuels and reduce greenhouse gas emissions.
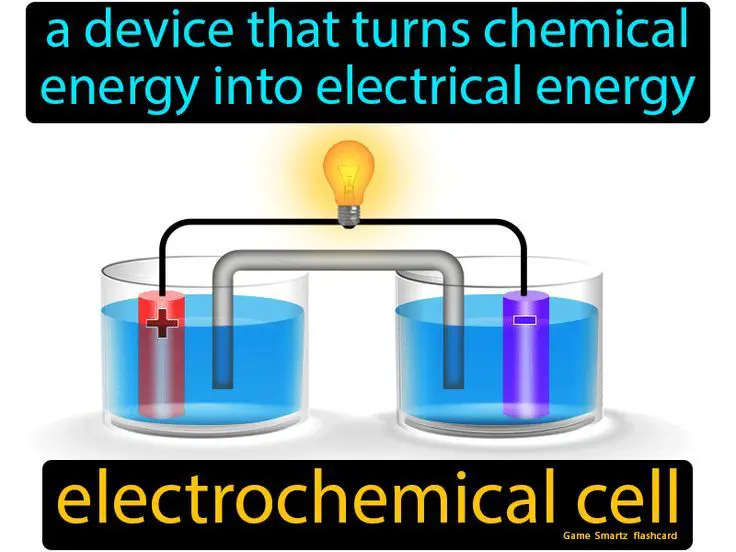
Electrochemical Reactions
Electrochemical reactions involve electron transfer between two substances, a reducing agent and an oxidizing agent. The most common type of electrochemical reactions used to produce electricity are redox reactions.
In a redox reaction, the reducing agent donates electrons to the oxidizing agent. This electron transfer generates an electric current that can be harnessed. Examples of redox reactions used in batteries include:
- Lead-acid battery: Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
- Lithium-ion battery: LiC6 + CoO2 ↔ LixCoO2 + C6
Fuel cells are electrochemical devices that convert the chemical energy of a fuel directly into electricity. They involve continuous redox reactions, where the fuel (reducing agent) and oxidant are supplied externally. Examples of fuels used include hydrogen, methane, and methanol.
Flow batteries are rechargeable batteries that store energy in external tanks in the form of two electrolyte solutions. These are pumped through an electrochemical cell where the redox reaction takes place, generating electricity. Flow batteries allow for larger energy storage capacities compared to solid electrode batteries.
Piezoelectric Effect
The piezoelectric effect refers to the ability of certain materials to generate an electric charge in response to applied mechanical stress. The word piezoelectricity means electricity resulting from pressure. It is an example of transducing energy from one form to another.
The piezoelectric effect occurs in crystalline materials that have no center of symmetry. When stress is applied, these materials develop a charge polarization from the displacement of ions. The polarization results in surface charges that can create a voltage between electrodes attached to the material. Conversely, applied voltage causes mechanical deformation or vibrations in the crystal structure.
The most common piezoelectric material is quartz. Piezoelectricity is also exhibited naturally by bone, tendon, wood, DNA, silk, and various proteins. It can also occur in man-made materials like ceramics and certain plastics.
Piezoelectric materials have important applications that rely on their ability to transduce energy. They can be used as sensors that detect changes in pressure, acceleration, temperature, strain or force by generating tiny amounts of voltage. They also have applications as actuators. When voltage is applied, they physically deform to create motion, sound or vibration.
Common applications of piezoelectricity include ultrasound and sonar devices, vibration sensors, igniters for lighters, microphone/guitar pickups, and fuel injector actuators for engines. Piezoelectric materials are also being developed for advanced applications like energy harvesting, vibration control, and micro-positioning devices.
Triboelectric Effect
The triboelectric effect refers to the phenomenon where certain materials become electrically charged after coming into contact and then separating from a different material. The charge transfer occurs due to the transfer of electrons between the surfaces of the two materials. Materials that have a greater tendency to give up electrons are said to be tribo-negative, while materials that readily accept electrons are tribo-positive.
When the two materials make contact, electrons transfer from the tribo-positive to the tribo-negative material. When the materials are separated, they retain net opposite charges. The triboelectric series ranks various materials by their tendency to gain or lose electrons. Materials higher on the series, like glass, tend to gain electrons when contacting materials lower on the series, like plastic.
The triboelectric effect can be harnessed to generate electricity through what is known as contact electrification or triboelectric nanogenerators (TENGs). In TENGs, the repeated contact and separation of two materials with opposite triboelectric polarities leads to an alternating flow of electrons between electrodes connected to the materials. The change in electric potential between the electrodes can be used to power devices. TENGs have advantages like high output, low cost, and excellent mechanical durability. They have applications for powering small electronics through harvesting ambient mechanical energy from motions like walking, tapping, or wind blowing.
Conclusion
In summary, there are several primary methods for transforming other forms of energy into electrical energy. The most common traditional methods rely on electromagnetic induction, where the movement of a magnet near a conductor induces a current, or the thermoelectric effect, where heat creates a voltage. More modern renewable methods utilize the photovoltaic effect, where light generates electricity in materials like silicon, or electrochemical reactions like in fuel cells and batteries. Other emerging methods take advantage of the piezoelectric and triboelectric effects, generating electricity from mechanical stress on certain materials.
As we seek to power the world in a cleaner and more sustainable way, renewable energy sources that transform natural energy flows like sunlight, wind, and water movement into usable electricity will only grow in importance. Though traditional electro-mechanical generators will continue to play a major role for the foreseeable future, supporting the transition to wind, solar, hydroelectric and other renewables is crucial to reducing our dependence on fossil fuels and building a greener energy infrastructure. Understanding how various forms of energy can be transformed into usable electricity will remain an essential scientific and engineering challenge.