How Does Fission Give Off Energy?
What is Nuclear Fission?
Nuclear fission is the process of splitting an atomic nucleus into smaller nuclei. During nuclear fission, the nucleus of an atom is bombarded with a neutron, causing it to split into two smaller nuclei and release energy in the form of heat and radiation.
This can be contrasted with nuclear fusion, where two smaller nuclei fuse together to create one larger nucleus, releasing tremendous amounts of energy in the process. While both nuclear fission and fusion release energy, they work in opposite directions – fission splits larger atoms while fusion combines smaller atoms.
Nuclear Fission Process
Nuclear fission is the process of splitting a heavy atomic nucleus like uranium into two lighter nuclei, which releases energy in the form of kinetic energy, binding energy, gamma rays, and neutrons. Fission occurs spontaneously with some heavier unstable isotopes such as Uranium-235, but can also be induced in other isotopes such as Uranium-238 by collision from a neutron.
The fission process begins when a neutron (which has no electrical charge) strikes the nucleus of a heavy atom like Uranium-235. This collision destabilizes the uranium nucleus and causes it to split into two smaller and lighter nuclei called fission fragments, plus 2 or 3 additional neutrons. The neutrons that are ejected can then strike other nearby uranium nuclei, causing them to split in a chain reaction that releases enormous amounts of energy.
When the uranium nucleus splits, its mass divides unevenly with most of the mass tightly binding together in the two fission fragments. However, according to Einstein’s famous equation E=mc^2, mass can be converted into energy. So around 0.1% of the original mass is converted into heat energy and electromagnetic energy in the form of gamma rays and kinetic energy in the then ejected neutrons.
Binding Energy
Binding energy is the minimum amount of energy required to disassemble the nucleus of an atom into its individual protons and neutrons. It is the amount of energy derived per nucleon if the atom were converted into pure neutrons.
Within the nucleus of an atom, protons and neutrons are held together by the nuclear strong force. When a pair of protons or neutrons are close together, they experience an attractive nuclear force, but when too far from each other they experience repulsion due to the electromagnetic charge of the proton. These competing forces produce a zone of stability where new additional energy is required to break the nucleus apart. The binding energy is a measure of the total energy that would be required to disassemble the nucleus.
In nuclear fission, a heavy nucleus like Uranium-235 or Plutonium-239 splits into two lighter nuclei, releasing neutrons and photons in the process. The sum of the masses of the resultant nuclei is less than the mass of the original heavy nucleus. This mass lost is converted into energy according to Einstein’s famous equation E=mc^2. Since the binding energy represents total energy holding the nucleus together, when this energy is released in fission it directly contributes to theuseful energy output that can be harnessed.
Mass Defect
Mass defect is the difference between the mass of an atomic nucleus and the total mass of its constituent protons and neutrons. This missing mass gets converted into nuclear binding energy that holds the nucleus together.
Based on Einstein’s mass-energy equivalence theory (E=mc2), this missing mass accounts for the large amounts of energy released during nuclear fission. When the nucleus splits into smaller nuclei, some matter is converted into energy. The more unstable the original nucleus was, the more binding energy will be released when it splits.
For example, when a Uranium-235 atom undergoes fission into Barium and Krypton, the mass defect is about 0.1 percent of the original mass. But when applied to Einstein’s equation, this converts to nearly 200 MeV of energy being given off per atom during the fission event. This demonstrates the enormous power contained within the atomic nucleus.
Kinetic Energy
One of the ways fission releases energy is in the form of kinetic energy of the fission fragments. When the uranium-235 nucleus splits, the fragments have high kinetic energy due to their high velocities. They are charged particles and slow down in the surrounding matter, losing energy due to collisions.The collisions eventually convert the energy of the fission fragments into the random motion that characterizes thermal energy or heat.
Chain Reaction
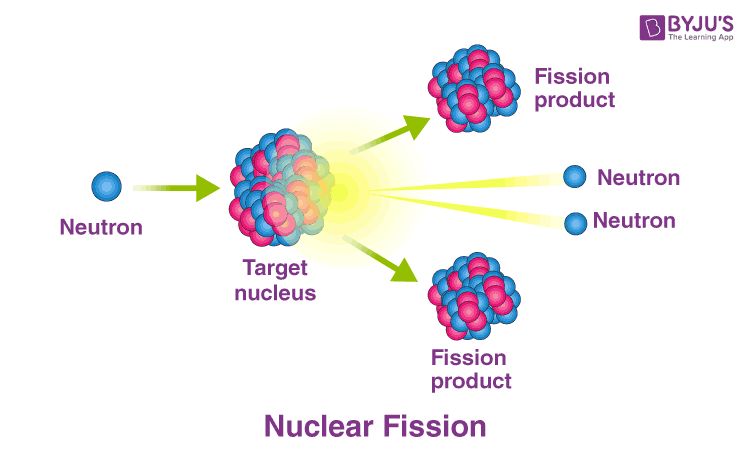
In nuclear fission, when a uranium atom is hit by a neutron, it splits into two fission fragments and two or three neutrons. These neutrons then go on to split more uranium atoms, releasing even more neutrons. This sets off a chain reaction. When a nuclear reactor achieves a critical mass of uranium fuel, it means there are enough unstable atoms being split to sustain a chain reaction. This critical mass of fuel and the chain reactions it creates are what produce energy in a nuclear reactor.
Controlled vs Uncontrolled
The nuclear fission process can become uncontrolled and dangerous when it occurs unregulated in nature or in unintentional human accidents. However, humans have learned to control nuclear fission in the following structured environments:
Controlled in nuclear reactors
Nuclear reactors are specially designed facilities where nuclear fission occurs in a safe, regulated manner. Instead of all the fission happening at once in an uncontrolled chain reaction, engineers use methods like control rods, moderators, and cooling systems to manage the rate and temperature of the fission reaction, harnessing its energy output to produce electricity on demand. Multiple redundant safeguards prevent accidents that would cause the reaction to get out of control.
Uncontrolled in nuclear bombs
Nuclear weapons use uncontrolled fission or fusion (or both) to create devastating explosions. The chain reaction happens extremely fast, nearly instantaneously, resulting in an unimaginable amount of energy released in fractions of a second. The components and purified fuel used in nuclear weapons are handled with extreme caution to prevent any unintentional criticality accidents.
Fission vs Fusion
Nuclear fission and fusion are two different types of reactions that release energy from atoms. The key differences between them are:
- Fission splits heavier atoms like uranium or plutonium into lighter atoms, fusion combines lighter atoms like hydrogen into heavier atoms.
- Fission reactions are easier to achieve technologically with current methods.
- Fusion releases 3-4x more energy per reaction than fission.
- Fission reactors generate radioactive waste that needs to be stored safely for long periods. Fusion reactors produce much less radioactive byproducts.
In terms of energy released, current fission reactors can reliably produce millions of kilowatt-hours of electricity from relatively small amounts of fuel. Fusion aims to be more efficient overall, but the technology to harness fusion energy is still in early experimental stages.
Safety Concerns
The use of nuclear fission for power generation and other applications introduces the challenge of safely controlling and disposing of radioactive waste.
Since radioactive materials remain harmful to human health for thousands of years, great care must be taken to securely store nuclear waste in repositories that prevent contamination of the environment.
Along with radioactive waste, the small but real risk of severe nuclear accidents also raises safety concerns. Human error, mechanical failure, and unforeseen events can all lead to catastrophic accidents that result in the spread of radiation. Nuclear facilities must employ multiple safeguards to greatly minimize these risks – examples include control rods, containment buildings, emergency core cooling systems, and more. Strict oversight and adherence to operating protocols is essential.
Future of Nuclear Power
Nuclear power has significant benefits as well as challenges going forward. On the benefit side, nuclear reactors produce large amounts of clean, carbon-free electricity on a well-established technology platform. Next generation reactors, such as small modular reactors and molten salt reactors, aim to improve upon the safety and waste challenges facing traditional large reactors while maintaining the same low-carbon benefits.
In terms of challenges, safety concerns still remain after high-profile incidents like Three Mile Island, Chernobyl and Fukushima. There is also the issue of cost and timeline – new nuclear plants are very expensive and take many years to build, while renewable energy costs have been falling very quickly. Finally, there is still no long-term solution for storing spent nuclear fuel waste.
The future direction and expansion of nuclear power likely depends on solving some of these key challenges through new technologies while emphasizing the benefits. For example, next generation molten salt and pebble bed reactors aim for inherent safety improvements over traditional reactors. New reactor designs utilizing alternative fuels and reprocessing may also help address nuclear waste concerns. Overall the technology holds promise but still faces public perception issues and economic competitiveness versus other generation sources.