How Does A Battery Generate Electrical Energy?
A battery is a device that converts chemical energy into electrical energy. It consists of one or more electrochemical cells, which contain positive and negative electrodes and an electrolyte solution. When the battery is connected to a device, chemical reactions occur that generate an electric current, allowing the battery to power the device.
Batteries serve as a portable source of power for a huge range of devices and applications. From consumer electronics like smartphones and laptops, to electric vehicles and large-scale energy storage systems, batteries allow electricity to be produced and stored for later use. This makes them an essential technology in the modern world.
The key components that make up a battery are the anode, cathode, electrolyte, and separators. The anode and cathode store chemical energy, while the electrolyte allows ions to flow between them. As chemical reactions occur, electrons flow from the anode to the cathode through an external circuit, producing electricity.
History
The history of batteries spans centuries, with early battery inventions focused on producing electricity through chemical reactions. In 1780, Italian scientist Luigi Galvani discovered that electricity could make a dead frog’s leg twitch when he touched it with two different metals. This finding revealed that electricity could be generated from chemical reactions. Building upon this, in 1800 Italian physicist Alessandro Volta invented the first true battery called the voltaic pile, which produced a continuous electric current. Volta’s battery consisted of alternating discs of zinc and copper separated by brine-soaked cloth or cardboard. This groundbreaking invention sparked developments that led to the wide variety of batteries we use today.
Battery Components
Batteries contain four main components that allow them to generate electricity:
Cathode
The cathode is the positive electrode in a battery. It is typically made of a metal oxide like manganese dioxide in alkaline batteries or lithium cobalt oxide in lithium-ion batteries. The cathode stores the positive charge during discharge and releases it during charging.
Anode
The anode is the negative electrode. It is usually made of a metal like zinc, lithium, or lead that can release electrons. The anode provides electrons to power the external circuit during discharge.
Electrolyte
The electrolyte is the conductive medium that allows ion flow between the cathode and anode. In alkaline batteries it is usually an alkaline paste of potassium hydroxide. In lithium batteries it is a lithium salt in an organic solvent.
Separator
The separator is a porous membrane that keeps the cathode and anode electrically isolated to prevent short circuits, while still allowing the electrolyte and ions to pass through. It is usually made of a material like polyethylene or polypropylene.
Chemical Reactions
Within a battery, chemical reactions generate electrons, allowing electrical current to flow through an external circuit. The key reactions are oxidation and reduction. In oxidation, atoms lose electrons. Reduction occurs when atoms gain electrons.
For example, in a typical zinc-carbon battery, zinc metal acts as the anode. Here, zinc atoms oxidize, lose electrons, and dissolve into the electrolyte as Zn2+ ions. The movement of these ions generates the electrical current that powers a device.
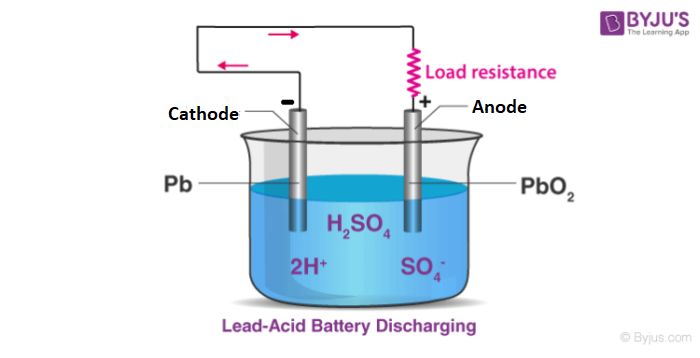
Meanwhile, the cathode is composed of manganese dioxide. This material gains electrons from the movement of zinc ions, undergoing reduction. The flow of negatively charged electrons through the external circuit balances the movement of positively charged zinc ions in the electrolyte.
The chemical potential difference between the anode and cathode materials drives the reactions. Electrons want to move from the anode (where they are generated by oxidation) to the cathode (where they are consumed by reduction). This electron flow is the electrical current that powers the battery.
Terminal Voltage
The terminal voltage, also known as electromotive force (EMF), is the potential difference between the positive and negative terminals of a battery when no current is flowing. This potential difference is what causes electrons to flow from the negative terminal, through the external circuit, and back to the positive terminal. The terminal voltage depends on the materials used in the battery electrodes and electrolytes.
In primary batteries, the terminal voltage is determined by the spontaneous chemical reactions occurring inside the battery between the anode and cathode materials. For example, an alkaline battery using zinc and manganese dioxide can produce around 1.5V. In secondary batteries, the terminal voltage is determined by the difference in chemical potential between the two electrodes.
When a load is connected to the battery, allowing current to flow, the terminal voltage will drop slightly due to internal resistance. But when no current flows, the terminal voltage indicates the maximum potential difference available from that battery.
Primary vs Secondary
Batteries are typically categorized as either primary or secondary batteries:
Primary batteries, also known as disposable batteries, are designed to be used once and discarded. They have a one-time use lifecycle and cannot be recharged. Common examples include alkaline batteries like AA and AAA batteries used in flashlights and remote controls. Primary batteries offer a burst of energy until their chemical reactants are depleted and then stop producing electricity.
Secondary batteries, also known as rechargeable batteries, can be recharged multiple times by applying an external electrical current which reverses the electrochemical reaction. This recharges the battery by replenishing the chemical reactants. Examples of secondary batteries include lithium-ion batteries used in consumer electronics and lead-acid batteries used in vehicles. Secondary batteries offer a renewable and more cost-effective source of power if properly recharged and maintained.
Both primary and secondary batteries convert chemical energy into electrical energy through redox reactions. However, their internal materials and reactions differ to optimize for either single-use or reusability. Choosing between disposable or rechargeable batteries depends on the application, cost factors, and ease of recharging.
Common Battery Types
There are several common types of batteries used today for various applications:
Alkaline Batteries
Alkaline batteries are common single-use batteries available in multiple standard sizes such as AAA, AA, C, D, and 9V. They contain zinc and manganese dioxide as the electrodes, and potassium hydroxide as the electrolyte. Alkaline batteries have a higher energy density than zinc-carbon batteries and can provide power longer in high-drain devices. However, they perform poorly in low temperatures.
Lithium-Ion Batteries
Lithium-ion (Li-ion) batteries are rechargeable batteries commonly used in consumer electronics like laptops and cellphones. They have high energy density and low self-discharge when not in use. Li-ion batteries use lithium cobalt oxide or lithium iron phosphate as the cathode and graphite as the anode. The electrolyte is an organic solvent containing lithium salts. Li-ion batteries can be dangerous if damaged or defective since they contain flammable electrolytes.
Lead-Acid Batteries
Lead-acid batteries are rechargeable batteries typically used in vehicles and backup power systems. The anode is metallic lead and the cathode is lead dioxide. The electrolyte is a sulfuric acid solution. Lead-acid batteries have a good capacity and power delivery but are very heavy compared to other rechargeable batteries. They also suffer capacity loss if drained completely.
Nickel-Cadmium Batteries
Nickel-cadmium (Ni-Cd) batteries are rechargeable batteries with nickel oxide hydroxide as the cathode and cadmium as the anode. The electrolyte is potassium hydroxide. Ni-Cd batteries have robust discharge characteristics and can deliver high currents. However, they contain toxic metals like cadmium and have been largely replaced by NiMH and Li-ion batteries.
Battery Capacity
Battery capacity refers to the amount of electrical charge a battery can deliver over its lifetime. It is typically measured in ampere-hours (Ah), which quantifies the total charge transferred at a constant current over a specified amount of time. The ampere-hour rating represents the maximum capacity a fully charged battery can provide.
Batteries with a higher ampere-hour rating can supply more current for longer periods before needing to be recharged. For example, a 12V car battery rated at 60Ah could theoretically deliver 60 amps for 1 hour, 30 amps for 2 hours, or any combination that multiplies to 60 amp-hours.
The C-rate represents the discharge rate relative to maximum capacity. A 1C discharge rate means discharging the battery’s full capacity in 1 hour. A 0.5C rate discharges the battery over 2 hours, while a 2C rate discharges it in 30 minutes. High discharge rates generate more heat and reduce overall lifespan.
Depth of discharge (DOD) measures the percentage of the total capacity that has been discharged during a cycle. Shallow discharges at 20-30% DOD are better for battery life than deep discharges down to 80-100% DOD. However, the usable capacity decreases at lower DOD.
Understanding these key metrics helps determine the appropriate battery for an application based on the required run-time, discharge rate, lifespan and usable capacity.
Safety Concerns
While batteries allow us to power countless devices, from phones to cars, they can also pose safety risks if improperly handled. Three main safety issues related to batteries are short circuits, overcharging, and thermal runaway.
Short Circuits
A short circuit occurs when the positive and negative terminals of a battery are connected directly, allowing excessive current to flow. This can generate significant heat and cause damage or even an explosion. To prevent short circuits, avoid storing batteries loosely where the terminals may contact metal objects or each other.
Overcharging
When batteries are charged beyond their maximum voltage, it causes electrolyte decomposition and internal pressure build-up. This can result in leakage or rupture. Using quality chargers that stop charging when the battery is full can prevent overcharging.
Thermal Runaway
If a battery severely overheats, it can cause a chain reaction leading to more overheating and eventually thermal runaway. This builds exponential heat until the battery catches fire or explodes. Properly storing batteries and avoiding damage that may lead to internal shorts helps prevent thermal runaway.
By taking proper precautions, the vast majority of battery safety risks can be avoided. Following manufacturer guidelines, using quality chargers, avoiding damage, and preventing short circuits are key to safe battery operation.
Battery Applications
Batteries power many of the devices we use every day. Some of the most common applications for batteries include:
Consumer Electronics
Small, portable batteries are essential for powering consumer electronics like cell phones, laptops, tablets, wireless headphones and speakers, and more. Different battery chemistries like lithium-ion are commonly used in these devices to provide sufficient energy density and voltage.
Vehicles
Rechargeable lead-acid batteries have long been used in vehicles to power the starter motor and electrical system. More recently, hybrid and electric vehicles use high capacity battery packs to store energy that powers the drivetrain. Lithium-ion batteries are commonly used in electric vehicles due to their high energy density.
Grid Storage
Large battery systems are increasingly being used to store energy on the electric grid. Grid storage batteries can help integrate renewable energy sources like wind and solar into the grid by storing excess energy when demand is low and discharging when demand is high. They also provide backup power and help stabilize the grid.