How Do You Make Electricity From Chemicals?
Generating electricity from chemicals, known as electrochemistry, is the process of creating electrical energy from chemical reactions. This occurs through redox reactions, where electrons are transferred between substances during oxidation and reduction. There are several ways to harness these electron transfers to produce an electric current and voltage that can power devices and circuits.
The most common electrochemical cells are batteries and fuel cells. In batteries, the reactants are stored within the cell and electricity is generated as those chemicals are consumed. Fuel cells require a continuous source of fuel and oxidant supplied from outside the cell. Other electrochemical electricity generation methods include thermocouples, photoelectrochemical cells, and microbial fuel cells.
This article will provide an overview of the history and main types of electrochemical electricity generation, explaining how each converts chemical energy into electrical energy through unique chemical reactions and processes.
History
The origins of generating electricity from chemical reactions date back to the late 18th and early 19th centuries with early experiments and discoveries made by scientists like Alessandro Volta, Michael Faraday, and Humphry Davy. In 1800, Volta invented the voltaic pile, one of the first batteries that produced a steady electric current, which led to a series of new experiments on electrochemistry. Building on Volta’s work, Faraday later conducted extensive research on electricity and magnetism in the 1820s and 1830s, discovering things like electromagnetic induction and electrolysis. Davy’s work isolating alkali metals through electrolysis of molten salts also expanded understanding of electrical currents from chemical reactions.
These pioneering scientists established the foundation of electrochemistry and demonstrated the possibility of generating electric currents via chemical reactions. Their discoveries opened up new possibilities for producing and storing electricity that would later evolve into commercial batteries, fuel cells, and other electrochemical technologies still used to this day.
Fuel Cells
Fuel cells are electrochemical devices that convert the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Fuel cells require continuous sources of fuel and oxygen to run, similar to how an internal combustion engine requires a continuous source of gasoline and air.
A fuel cell consists of two electrodes, the anode and cathode, separated by an electrolyte. Different types of fuel cells are categorized by the kind of electrolyte they use. Common electrolytes include potassium hydroxide, phosphoric acid, molten carbonate, and solid oxides.
At the anode, the fuel (often hydrogen) is oxidized, releasing electrons. The electrons travel through an external circuit, creating electricity to power devices. Meanwhile, at the cathode, oxygen is reduced, consuming electrons. The electrons travel through the external circuit from the anode to the cathode, creating an electrical current.
Some key types of fuel cells include:
- Proton exchange membrane fuel cells (PEMFC) – Use a solid polymer electrolyte and porous carbon electrodes. Commonly used in vehicles and portable applications.
- Phosphoric acid fuel cells (PAFC) – Use liquid phosphoric acid as the electrolyte. Mainly used for stationary power generation.
- Molten carbonate fuel cells (MCFC) – High temperature cells with molten carbonate salt electrolyte. Used for power generation applications.
- Solid oxide fuel cells (SOFC) – Use a solid ceramic electrolyte. Operate at very high temperatures around 1,000°C. Used for stationary and transportation purposes.
Compared to combustion engines, fuel cells have higher efficiency with less noise and few moving parts. However, durability issues and high costs have limited the widespread commercialization of fuel cell technologies. Ongoing research aims to improve the performance and affordability of fuel cells.
Batteries
Batteries are electrochemical devices that convert stored chemical energy into electrical energy. They consist of one or more electrochemical cells, each containing a positive electrode (cathode), a negative electrode (anode), and an electrolyte that allows ions to move between the electrodes.
The cathode and anode are made of different chemical compounds. In primary (non-rechargeable) batteries, the reaction is irreversible and the battery discharges over time as the chemical reactants are depleted. In secondary (rechargeable) batteries, the chemical reaction can be reversed by running an electrical current into the battery to recharge it.
Some common battery types include:
- Lead acid batteries – Used in automobiles, these contain lead dioxide (cathode) and metallic lead (anode), with sulfuric acid as the electrolyte.
- Lithium ion batteries – Very common in consumer electronics, these batteries contain lithiated metal oxide cathodes, graphite anodes, and lithium salt electrolytes.
- Alkaline batteries – Disposable primary batteries using zinc (anode) and manganese dioxide (cathode), with an alkaline electrolyte.
- Nickel-cadmium – Rechargeable batteries with nickel oxide hydroxide (cathode), metallic cadmium (anode) and potassium hydroxide electrolyte.
When a battery discharges, oxidation reactions occur at the anode, producing electrons that flow through the external circuit. The electrons then drive reduction reactions at the cathode. This electron flow generates electricity that can be harnessed.
Electrochemistry
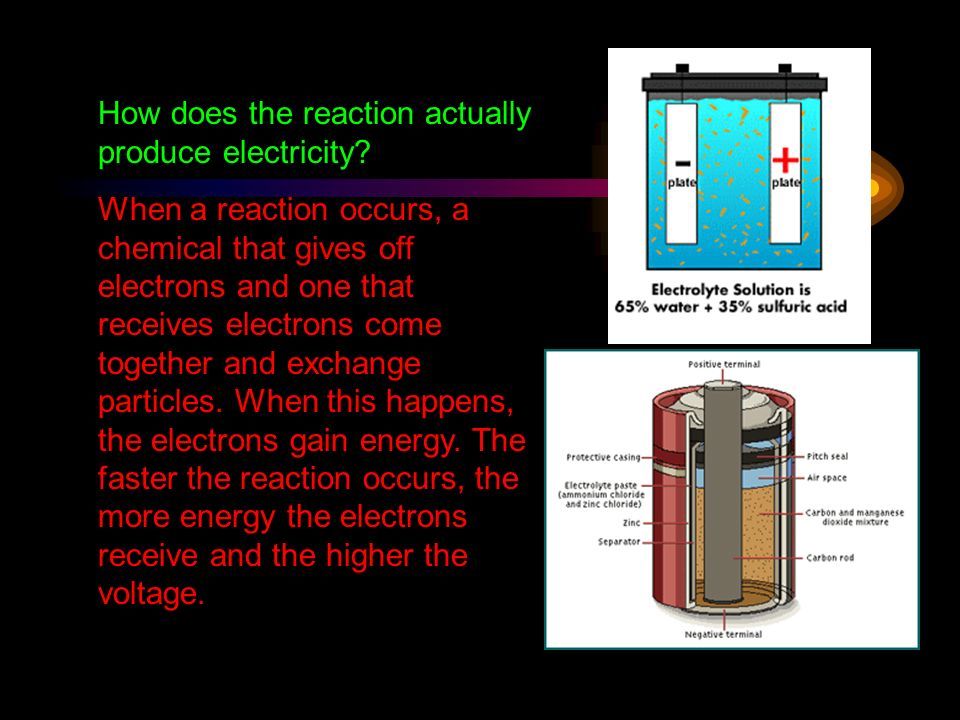
Electrochemistry involves oxidation-reduction reactions which produce an electric current from chemical reactions. In an oxidation-reduction reaction, electrons are transferred between chemicals. One chemical is oxidized, meaning it loses electrons, while the other chemical is reduced, meaning it gains electrons.
For example, a common redox reaction occurs inside batteries. The anode gives up electrons to the cathode, generating a flow of electrons from the anode to the cathode. This flow of electrons is an electric current that can be captured and utilized.
Ions may also move in an oxidation-reduction reaction. Positively charged cations migrate toward the cathode, while negatively charged anions migrate toward the anode. This ion flow occurs in solution or in the solid state.
The separation of charges that occurs in a redox reaction allows the electric current to be harnessed. Electrochemistry leverages these chemical reactions to generate electricity for practical applications. Fuel cells, batteries, and other electrochemical cells all rely on the principles of electrochemistry to convert chemical energy into useful electrical energy.
Thermocouples
Thermocouples are devices that convert heat directly into electricity using the Seebeck effect. The Seebeck effect refers to when a voltage is generated between two dissimilar electrical conductors or semiconductors that are subject to a temperature gradient.
Thermoelectric materials that produce a large voltage for a given temperature gradient are essential for thermocouples. Semiconductors like bismuth telluride are commonly used. Metals can also be used, but semiconductors typically have a larger Seebeck coefficient. The materials used depend on the operating temperature range.
Some applications of thermocouples include temperature measurement, power generation, and heating and cooling equipment. For temperature measurement, thermocouples can measure a wide range of temperatures by correlating the voltage produced to temperature. For power generation, the heat from burning fuel or waste heat can be converted into electricity. In heating and cooling devices, applying a current reverses the Seebeck effect to pump heat.
Photoelectrochemical Cells
Photoelectrochemical cells convert solar energy into electrical energy through the photovoltaic effect. They use semiconducting materials called photocatalysts that produce an electric current when exposed to sunlight.
When sunlight hits the photocatalyst, the photons excite electrons in the material to higher energy states. This frees up electrons to move through an external circuit and generate electricity. The most common photocatalyst is titanium dioxide, which is inexpensive, stable, and nontoxic.
The photoexcited electrons flow from the photocatalyst to a counter electrode through an external circuit. This flow of electrons is the electric current. Meanwhile, the “holes” left behind by electrons in the photocatalyst attract electrons from a sacrificial electron donor or a redox couple electrolyte. This completes the circuit.
Photoelectrochemical cells can be more efficient at converting solar energy than passive semiconductor devices. They also separate the light absorption and charge carrier transport steps, allowing each component to be optimized. This makes them a promising technology for sustainable solar energy conversion.
Microbial Fuel Cells
Microbial fuel cells (MFCs) use bacteria to convert organic matter into electricity. The bacteria act as a biocatalyst to break down organic matter and release electrons that can be captured as an electrical current.
Bacterial metabolism is at the heart of MFCs. Certain bacteria can digest organic matter like glucose or wastewater through anaerobic respiration. This process breaks down the organic matter and generates protons and electrons as byproducts.
The MFC setup allows for the capture of the released electrons on an anode. The electrons then flow through a circuit to the cathode, generating an electrical current. Oxygen is reduced at the cathode. Meanwhile, the protons pass through a membrane to the cathode where they combine with oxygen and the electrons to produce water.
MFCs have useful applications for renewable energy production and wastewater treatment. They can generate electricity from the organic matter in wastewater. The organic matter gets depleted in the process, helping to clean the wastewater. MFCs are also being researched for power generation from other organic matter sources like food processing waste, agricultural runoff, and even insect larvae.
MFCs offer a sustainable way to simultaneously generate electricity and treat organic waste. With further development, they have potential for decentralized energy production and low-cost wastewater treatment.
Piezoelectricity
Piezoelectricity refers to the ability of certain materials to generate an electric charge in response to applied mechanical stress. The word piezoelectricity means electricity resulting from pressure. It is an example of converting mechanical energy into electrical energy on an atomic scale.
Piezoelectric materials exhibit a lattice structure such that the positive and negative centers of charge within the crystal do not coincide. When stress is applied, the ions shift slightly, resulting in a net polarization and rearrangement of the dipole moments within the crystal structure. This polarization results in a voltage across the material. The amount of voltage generated depends on the amount of mechanical stress applied.
Some naturally piezoelectric materials include quartz, Rochelle salt, topaz, tourmaline, and bone. Many piezoelectric materials used today are man-made, like barium titanate or lead zirconate titanate (PZT). The piezoelectric effect is reversible – applying an electric field will cause the material to mechanically deform or vibrate. This allows piezoelectric materials to be used both as sensors and actuators.
Piezoelectric materials have many applications that take advantage of their ability to convert mechanical energy to electrical energy. They are used in pressure sensors, sonar devices, buzzers, and ultrasound devices. They can also be used to generate high voltages needed for technologies like spark plugs or plasma production.
Conclusion
Throughout history, humans have harnessed various chemical reactions to produce electricity for powering devices and equipment. From primitive batteries to fuel cells and thermocouples, electrochemistry has enabled the generation of electrical current from chemical energy sources.
While existing technologies like batteries and fuel cells continue to improve in efficiency and capacity, the future points to new methods and materials for chemical electricity production. Microbial fuel cells leverage bacteria to generate power, while advances in materials science have enabled electricity generation from mechanical stress on crystalline materials via the piezoelectric effect.
As the world shifts to renewable and sustainable energy, electrochemical electricity production will likely play a key role. With further research and development, chemicals and materials may unlock new and exciting possibilities for clean, efficient energy to power our future.