How Do You Differentiate Between Matter And Energy And Between Potential And Kinetic Energy?
Defining Matter
Matter is anything that has mass and takes up space. All physical objects are made up of matter. Matter is composed of tiny particles called atoms and molecules that are too small to be seen by the naked eye. Atoms are the basic building blocks of all matter and molecules are groups of atoms bonded together. For example, a grain of salt contains trillions of atoms bonded together to form a single molecule. All matter, regardless of its size or type, is made up of atoms in different combinations and arrangements. The mass and volume that an object possesses comes from the atoms and molecules that compose it. Anything that has mass and volume, from a pebble to a skyscraper, is considered matter.
Types of Matter
Matter exists in four fundamental states: solid, liquid, gas, and plasma. Each state has unique properties:
Solids have a defined shape and volume. The molecules are packed tightly together and can only vibrate in place. Solids cannot be compressed.
Liquids have a defined volume but take the shape of their container. The molecules flow freely past each other. Liquids are difficult to compress.
Gases do not have a defined shape or volume; they expand to fill their container. The molecules move freely and are highly compressible.
Plasma is a high-energy state of matter where the atoms are ionized. Plasma does not have a definite shape or volume. Examples include lightning and the glowing matter inside fluorescent light bulbs.
Defining Energy
Energy is the ability to do work or produce heat. It is a quantitative property that exists as potential or kinetic forms. Energy cannot be created or destroyed, only transformed from one form into another. For example, a battery stores chemical energy that can be transformed into electrical energy to power a flashlight. Or gasoline contains chemical energy that can be transformed into kinetic energy to propel a car forward. The law of conservation of energy states that the total energy in an isolated system remains constant.
There are many different types of energy, but they can be broadly categorized into two main forms – potential energy and kinetic energy. Potential energy is stored energy that has the potential to do work. Kinetic energy is energy of motion that is doing work. Understanding the difference between these two forms of energy is important across many scientific fields.
Types of Energy
Energy comes in many different forms that can be categorized into two main types: potential energy and kinetic energy. Potential energy is stored energy that has the potential to be used, while kinetic energy is energy in motion. Here are some of the most common forms of energy:
- Potential Energy
- Chemical Energy – Energy stored in the bonds between atoms and molecules. Examples are batteries, food, fuel.
- Nuclear Energy – Energy stored in the nucleus of an atom. Examples are nuclear power plants, nuclear weapons.
- Gravitational Potential Energy – Energy stored by virtue of position or height. Examples are water behind a dam, object on a shelf.
- Elastic Potential Energy – Energy stored in elastic materials that are stretched or compressed. Examples are stretched springs, rubber bands.
- Kinetic Energy
- Radiant Energy – Electromagnetic energy that travels in waves. Examples are microwaves, radio waves, light.
- Thermal Energy – The internal energy of an object arising from the motion of atoms and molecules. Basically, the energy of heat.
- Motion Energy – The energy possessed by an object in motion. Examples are cars driving, people walking.
- Sound Energy – Caused by the back-and-forth vibration of particles. The energy carried by sound waves.
Potential vs Kinetic Energy
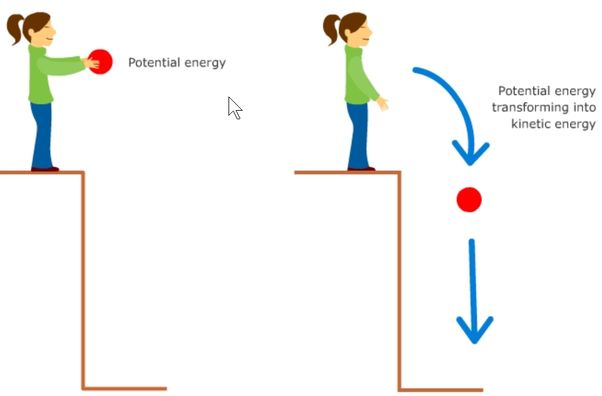
Potential energy is stored energy based on an object’s position or arrangement. For example, a ball held at a height above the ground has potential energy due to gravity. When the ball is dropped, this potential energy is converted into kinetic energy, which is the energy of motion. The ball accelerates as it falls, gaining kinetic energy until it hits the ground.
Other examples of potential energy include a compressed spring, stretched elastic, chemical energy in batteries, and nuclear energy in atoms. In each case, there is energy ready to be released and do work. Kinetic energy, on the other hand, is the energy an object has due to its motion. A moving object can do work by colliding and pushing other objects. Kinetic energy depends on the object’s mass and velocity – the heavier and faster an object moves, the more kinetic energy it has.
Energy can convert between potential and kinetic forms. As the ball falls, its potential energy decreases while its kinetic energy increases. Understanding the difference between potential and kinetic energy is important in physics, engineering, and other fields.
Converting Between Potential and Kinetic
Energy can convert between potential and kinetic forms, but the total amount of energy remains constant. This is known as the law of conservation of energy. For example, when an object falls, its potential energy decreases while its kinetic energy increases. As the object falls, gravity does work on the object, transferring energy from potential to kinetic. The formula for potential energy depends on the situation, but commonly involves mass, gravity, and height. The formula for kinetic energy is 1/2*mass*velocity^2. So as velocity increases due to the fall, kinetic energy increases while potential energy decreases. But the total energy at the start when the object is stationary equals the total energy at any point during the fall. Energy is never lost, just converted between different forms.
Examples of Potential Energy
There are several common types of potential energy:
- Gravitational potential energy – This is energy stored due to an object’s height above the ground. For example, a book sitting on a desk has more gravitational potential energy than a book lying on the floor. As the book falls, its potential energy converts into kinetic energy.
- Elastic potential energy – Energy stored in elastic materials that are stretched or compressed. For example, a stretched rubber band or compressed spring contains elastic potential energy. This energy gets released when the tension is released.
- Chemical potential energy – Energy stored in the bonds between atoms and molecules. This energy gets released in chemical reactions. Common examples include batteries, food, fuel, and explosives.
- Nuclear potential energy – Energy stored in the nucleus of an atom, related to the binding energy between nucleons. Nuclear potential energy gets released in nuclear reactions, including radioactive decay, nuclear fission, and nuclear fusion.
Understanding the various forms of potential energy allows us to harness them for practical uses. Potential energy plays an important role across many engineering and technological systems.
Examples of Kinetic Energy
Some common examples of kinetic energy include:
– The energy of moving objects. A moving car, truck, train, or plane all have kinetic energy. The faster an object moves, the more kinetic energy it has.
– Flowing water. The movement of rivers, waterfalls, ocean waves, and rainfall all involve kinetic energy.
– Heat. On a molecular level, heat consists of atoms and molecules vibrating and moving. The greater the molecular motion, the hotter something is, indicating more kinetic energy.
– Light. Light waves and photons exhibit kinetic energy as they travel. The energy in light allows it to do work, such as enabling photosynthesis in plants.
– Electrical energy. The movement of electrons in electrical currents involves kinetic energy. This energy can be harnessed to power devices and equipment.
– Sound. The vibrations that create sound waves contain kinetic energy. This energy dissipates as sound travels through the air.
In short, kinetic energy represents the active, moving form of energy. Any objects, substances, or phenomena that involve motion contain this type of energy.
Significance of Potential vs Kinetic Energy
Understanding the interconversion between potential and kinetic energy has many important real-world applications. One of the most significant is in the generation of hydroelectric power. Dams are built to store water at a height, which gives it gravitational potential energy. When the water is released through turbines, this potential energy gets converted into kinetic energy to rotate the turbines and generate electricity. Other applications that rely on converting between potential and kinetic energy include pendulums, rollercoasters, and springs.
Knowing how to interconvert potential and kinetic energy allows engineers and designers to build systems that efficiently capture energy in a potential form, store it, and then release it in a controlled way to do useful work. This concept is key for renewable energy sources like hydro, wind, and solar power. Overall, the ability to convert between potential and kinetic energy makes it possible to generate electricity, power machinery, enable transportation, and utilize many technologies that are essential in the modern world.
Key Takeaways
Matter refers to anything that has mass and takes up space, while energy refers to the ability to do work or produce heat. The main types of matter are solids, liquids, gases, and plasma. The main types of energy are potential energy, kinetic energy, thermal energy, chemical energy, nuclear energy, electrical energy, radiant energy, and mechanical energy.
Potential energy refers to stored energy based on an object’s position or arrangement, while kinetic energy refers to energy associated with motion. For example, a ball at the top of a ramp has potential energy, while a ball rolling down the ramp has kinetic energy. The potential energy can be converted into kinetic energy as the ball rolls down. Understanding the difference between potential and kinetic energy is important across many fields, from physics to engineering and more.
Key takeaways include:
- Matter occupies space and has mass, while energy is the ability to do work or produce heat.
- The main types of matter are solids, liquids, gases and plasma. The main types of energy include potential, kinetic, thermal, chemical, nuclear, electrical, radiant and mechanical.
- Potential energy depends on an object’s position or arrangement. Kinetic energy is energy associated with motion.
- Potential energy can convert into kinetic energy, like a ball rolling down a ramp.
- The ability to differentiate between potential and kinetic energy is important for physics, engineering and more.