Does Thermal Energy Depend On Quantity?
Thermal energy, often referred to as heat energy, is the energy possessed by an object or system due to the motion of its atoms and molecules. It is essentially the internal kinetic energy of an object’s molecules that results in its temperature. Thermal energy flows from objects at higher temperatures to objects at lower temperatures until thermal equilibrium is reached.
This article will provide an overview of thermal energy, including how it is measured, the factors that affect it, how it relates to phase changes, and whether the quantity of matter affects the thermal energy.
Thermal Energy Basics
Thermal energy refers to the total kinetic energy of all the molecules within an object. This energy is associated with the motions and vibrations of the atoms and molecules that make up that matter. The higher the thermal energy of an object, the more active the molecules are within it.
Thermal energy is related to, but distinct from, heat and temperature. Heat is the transfer of thermal energy between objects, while temperature measures the average kinetic energy of molecules within an object. Objects with more heat don’t necessarily have more thermal energy overall. However, increasing thermal energy generally raises the temperature.
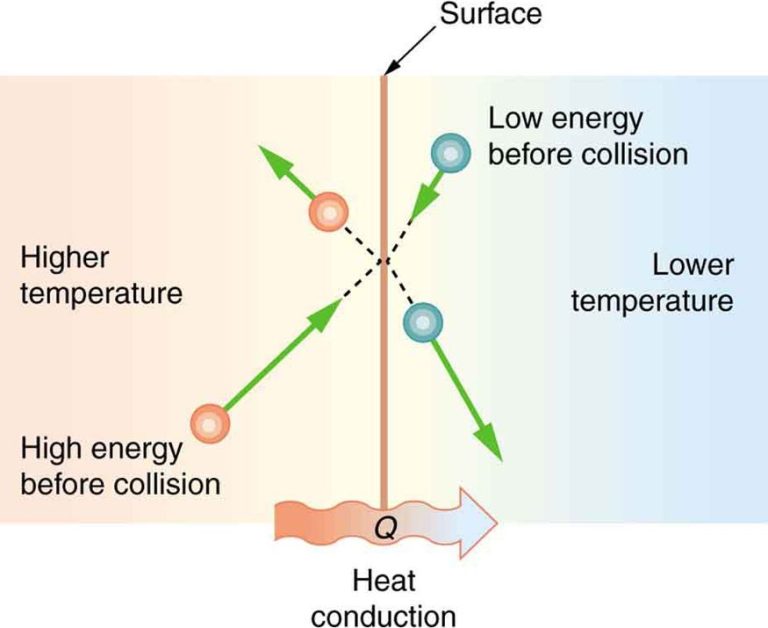
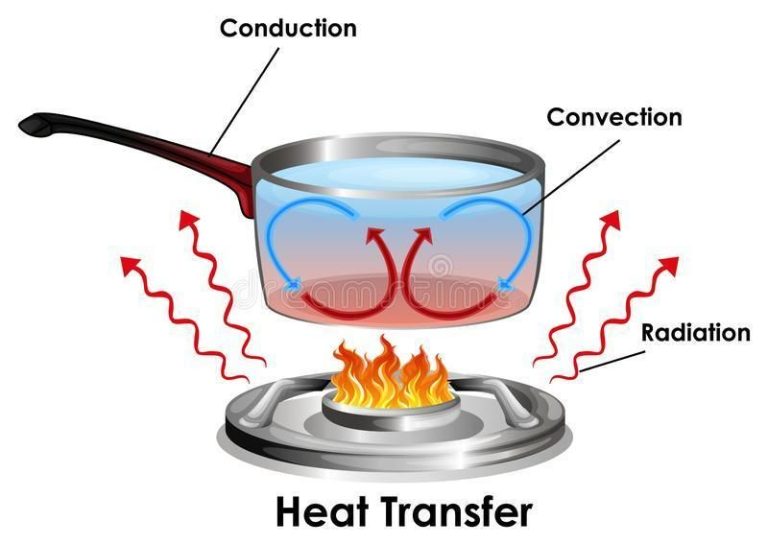
Thermal energy flows spontaneously from objects at higher temperatures to objects at lower temperatures. This transfer of energy continues until thermal equilibrium is reached and the objects are at the same temperature. The methods of heat transfer are conduction, convection, and radiation.
Measuring Thermal Energy
Thermal energy is quantified and measured using units called joules or calories. A joule is defined as the amount of energy transferred when a force of one newton displaces an object one meter. A calorie is defined as the amount of energy required to raise one gram of water by one degree Celsius.
On a microscopic scale, thermal energy comes from the kinetic energy of atoms and molecules. As an object gets hotter, its atoms and molecules vibrate and move faster, resulting in an increase in thermal energy. Measuring devices like thermometers and calorimeters allow us to quantify thermal energy on a macroscopic scale.
Thermometers measure temperature, which correlates to the average kinetic energy of particles in a substance. Calorimeters allow us to directly measure the amount of heat energy absorbed or released during a physical or chemical change. Sophisticated instruments like bomb calorimeters can precisely measure the energy content in chemical samples.
By utilizing standardized units and measurement tools, we can put numeric values on the thermal energy in a given system or sample. This allows us to quantitatively study how thermal energy is transferred during heating and cooling processes.
Factors Affecting Thermal Energy
There are three main factors that determine the thermal energy of an object: mass, specific heat capacity, and temperature change.

Mass refers to the amount of matter in an object. The more massive an object is, the more atoms and molecules it contains, and therefore the more thermal energy it possesses at a given temperature. For example, 1 kg of water has more thermal energy than 0.5 kg of water at the same temperature.
Specific heat capacity is a property of the material or substance. It measures how much energy is needed to raise the temperature of 1 kg of a substance by 1°C. Substances like water or iron have high specific heat capacities, so they require more energy to change temperature compared to substances with low specific heat capacities.
Temperature change refers to the difference between the initial and final temperatures of an object. The greater the temperature change, the more thermal energy is gained or lost. For example, heating water from 20°C to 80°C involves a greater temperature change and energy transfer than heating it from 20°C to 30°C.
The relationship between these three factors is described by the equation: Q = mcΔT, where Q is thermal energy, m is mass, c is specific heat capacity, and ΔT is the temperature change. Therefore, the thermal energy of an object depends on all three of these variables – mass, specific heat, and temperature change.
Thermal Energy of Identical Objects
When comparing two identical objects, the object with the greater mass will possess more thermal energy. This is because thermal energy is directly proportional to the mass for a given material at the same temperature.
For example, consider two cast iron frying pans at 200°C. Frying pan A has a mass of 2 kg. Frying pan B has a mass of 4 kg. Since both frying pans are made of the same material (cast iron) and are at the same temperature, the frying pan with more mass (pan B) will have more thermal energy.
Specifically, if we calculate the thermal energy of each frying pan using the formula:
Thermal Energy = Specific Heat Capacity x Mass x Temperature Change
We find:
Frying Pan A = 0.45 kJ/kg°C x 2 kg x 200°C = 180 kJ
Frying Pan B = 0.45 kJ/kg°C x 4 kg x 200°C = 360 kJ
As shown, the 4 kg frying pan has twice as much thermal energy as the 2 kg frying pan, because it has twice the mass. This demonstrates that for identical objects, thermal energy scales linearly with mass.
Thermal Energy of Different Materials
The amount of thermal energy stored in an object depends not just on its mass, but also the type of material it is made of. This is because different materials have different capacities for absorbing and storing heat energy. The specific heat capacity of a substance measures how much energy is required to raise the temperature of 1 gram of that substance by 1 degree Celsius.
Substances like metals generally have lower specific heat capacities, meaning less energy is required to change their temperature. Materials like water have very high specific heat capacities, requiring more energy to induce a temperature change. This means that for two objects of identical mass, the one made of the material with higher specific heat capacity will be able to absorb and store more thermal energy.
For example, imagine two cast iron and aluminum cubes of identical 1 kg mass. It takes approximately 449 J to raise the temperature of 1 gram of iron by 1°C. For aluminum, it only takes about 903 J to raise 1 gram by 1°C. Therefore, if both cubes were heated by the same amount, the aluminum cube would reach a higher temperature because aluminum has a lower specific heat capacity. The iron cube would absorb and retain more thermal energy overall at the same temperature.
Thermal Energy and Phase Changes
Thermal energy is absorbed or released when a substance undergoes a phase change, such as melting, freezing, boiling, condensing, or sublimating. These phase changes occur because they require energy to break or form the bonds between molecules.
For example, when water freezes into ice, the water molecules slow down and form rigid crystal structures. The process requires thermal energy to break some of the hydrogen bonds between water molecules. This thermal energy is released into the surroundings, resulting in a decrease in thermal energy of the substance.
On the other hand, when ice melts into liquid water, thermal energy must be absorbed from the surroundings to increase the molecular motion and break apart the crystal structure of ice. This absorption of thermal energy during melting results in an increase in the thermal energy of the substance.
The amount of thermal energy absorbed or released depends on the amount of substance undergoing the phase change. Therefore, more thermal energy is required to melt a large ice cube compared to a small one. Each substance has a specific latent heat capacity associated with each phase transition, which quantifies this energy transfer.
Applications and Examples
Thermal energy concepts show up in many real-world applications and examples:
When boiling water to cook pasta, the heat from the stove increases the thermal energy of the pot and water, allowing the water to reach its boiling point. The quantity of water determines how much thermal energy is needed to bring it to a boil. More water requires more thermal energy input.
Spacecrafts re-entering the atmosphere experience intense heating as kinetic energy is converted into thermal energy due to drag and friction with the air. Thermal protection systems are designed to withstand high temperatures and insulate the spacecraft. The larger the spacecraft, the more thermal energy it will have to dissipate.
Insulating a house conserves thermal energy by reducing conduction, convection, and radiation. The amount of insulation needed depends on the size of the house, with larger houses requiring more insulation material to adequately conserve the thermal energy inside.
Solar water heating systems rely on the sun’s thermal energy to heat water. The size of the storage tank and solar collector area impacts the quantity of hot water that can be produced. Larger systems can provide more hot water for applications like heating a swimming pool.
Thermal power plants use sources like coal, natural gas, or nuclear fuel to heat water and produce steam that spins a turbine to generate electricity. Larger power plants have greater energy output as they burn more fuel to release more thermal energy.
Does Quantity Affect Thermal Energy?
When examining how quantity affects thermal energy, we need to consider a few key factors:
Identical substances: If we have two identical substances with different quantities, their thermal energy will be proportional to their quantity. For example, 1 kg of water at 20°C will have half the thermal energy of 2 kg of water at 20°C. So for identical substances, thermal energy does depend directly on quantity.
Different materials: If we compare different materials, quantity affects thermal energy differently. 1 kg of water may have a different thermal energy than 1 kg of iron at the same temperature. This is because substances have different heat capacities. So for different materials, thermal energy depends on both quantity and the specific heat capacity of the material.
Phase changes: Changing phase requires energy, known as latent heat. The amount of energy required for a phase change depends only on the material and mass, not the quantity. For example, 1 kg of water requires the same latent heat to boil as 10 kg of water.
In summary, quantity directly affects thermal energy only for identical substances. When materials differ, thermal energy depends on both mass and the specific heat capacity of each material. Phase changes depend only on the material and mass involved, not the quantity.
Conclusion
Thermal energy is an important concept in physics and engineering. This article provided an overview of the basics of thermal energy, factors that affect it, and how it relates to quantity.
Key points covered include:
- Thermal energy refers to the total kinetic energy of molecules in a substance. It depends on the temperature, mass, and composition of the substance.
- Measuring thermal energy requires understanding heat capacity, temperature change, and phase changes.
- The thermal energy of identical objects depends on their mass – more massive objects contain more thermal energy at the same temperature.
- Different materials have different heat capacities, affecting how much thermal energy they hold.
- Phase changes require large amounts of thermal energy, as molecules rearrange during melting, boiling, etc.
Regarding how quantity affects thermal energy, the analysis showed that greater quantities of a substance contain proportionally greater thermal energy at the same temperature. However, intensive factors like temperature and specific heat capacity determine the thermal energy per unit mass.
In summary, while thermal energy does depend on quantity, the relationship also depends on material properties and temperature. This knowledge has important applications in science and engineering.