Can You Turn Kinetic Energy Into Heat?
What is Kinetic Energy?
Kinetic energy is defined as the energy associated with motion. An object that has motion – whether it is vertical or horizontal motion – has kinetic energy. The amount of kinetic energy depends on the mass and velocity of the object. The more massive the object and the faster it is moving, the more kinetic energy it possesses.
We encounter kinetic energy frequently in everyday life. Some common examples include:
- A moving car
- A bicycle in motion
- Basketballs bouncing
- Leaves blowing in the wind
- A flowing river or stream
In each case, the object in motion has kinetic energy. Kinetic energy allows the object to do work and transfer energy into other forms. Understanding the kinetic energy possessed by various objects helps explain many physical phenomena we observe.
Relationship Between Kinetic Energy and Speed
There is an exponential relationship between the kinetic energy of an object and its speed. This means that a small increase in speed can lead to a large increase in kinetic energy. Specifically, kinetic energy is proportional to the square of an object’s speed.
Kinetic energy can be calculated using the following formula:
Kinetic Energy = 1/2 x mass x velocity^2
So if an object’s mass stays constant, but its velocity doubles, its kinetic energy will increase by a factor of 4. For example, if a 1kg object moves at 2 m/s, it has 2 Joules of kinetic energy. If its speed doubles to 4 m/s, its kinetic energy increases to 16 Joules.
This exponential relationship between speed and kinetic energy is very important. It explains why even small increases in velocity can greatly increase the impact force and damage caused by fast moving objects. For example, this is why car safety features like airbags and seatbelts are so important. At highway speeds, a collision has dramatically higher kinetic energy than at lower speeds, and this energy needs to be managed to prevent injury.
Generating Heat Through Friction

One of the most common ways that kinetic energy gets converted into heat is through friction. Friction is a force that occurs when two surfaces rub against each other. The friction causes the kinetic energy of the motion to be converted into thermal energy, which we perceive as heat.
A simple example of this is when you rub your hands together quickly. The kinetic energy of your moving hands is converted by the friction between your palms into heat. This heat generated by friction is why your hands feel warmer after rubbing them together. The faster you rub your hands, the more kinetic energy is present, and the more heat is produced.
Other Ways to Convert Kinetic Energy to Heat
In addition to friction, there are other processes that can convert the kinetic energy of motion into thermal energy in the form of heat. Some key examples include:
Deformation and Compression
When an object is compressed or deformed, work is done to change its shape. This exerts internal stresses on the material that manifest as heat. For example, when jumping and landing, the compression of leg muscles and tendons converts kinetic energy into thermal energy.
Turbulence and Fluid Friction
When fluid flows encounter obstacles or roughness, eddies and turbulence can develop. The kinetic energy of the bulk fluid motion gets converted into the random microscopic motion of turbulence, heating the fluid through viscous friction.
Internal Friction in Solids
Solids also experience internal friction when subjected to stresses and deformation. The kinetic vibrational energy of atoms gets converted into heat through interatomic collisions and damping. This effect depends on the material and is especially pronounced in viscoelastic polymers.
Heat Transfer Mechanisms
There are three main mechanisms by which heat transfers from one object or system to another: conduction, convection, and radiation.
Conduction is the transfer of heat between substances that are in direct contact with each other. It occurs when heat flows from the hotter end to the colder end of an object until temperature equilibrium is reached. The rate of conductive heat transfer depends on the temperature difference and the thermal conductivity of the materials involved. Metals like copper and aluminum are good conductors while non-metals like glass and wood are poor conductors.
Convection is the transfer of heat by the movement of hot and cold substances. It occurs in gases and liquids, where hot regions become less dense and rise while cold regions sink. This sets up circulation currents that transfer heat around. Convection depends on various factors like temperature gradients and fluid velocities. Common examples include heating and cooling through air currents or hot water rising.
Radiation is the transfer of heat via electromagnetic waves or photons. It does not require direct contact between substances. All objects emit and absorb radiation depending on their temperature. The hotter an object, the more radiation it emits. Radiant heat can travel long distances through vacuums. The warmth of the sun reaching Earth is an example of radiative heat transfer.
Applications and Examples
There are many real-world applications that demonstrate the conversion of kinetic energy into heat energy through friction and other means. Two common examples are car brakes and meteorites entering the earth’s atmosphere.
Car brakes use friction to rapidly convert the vehicle’s kinetic energy into heat. When the brake pads press against the rotating brake rotor, friction causes the components to heat up rapidly, slowing the vehicle down. The kinetic energy of the moving car gets dissipated as heat. This allows the car to safely come to a stop. Without this conversion of motion into heat, the brakes would not be able to stop the vehicle.
Meteorites entering earth’s atmosphere also demonstrate the conversion of kinetic energy into thermal energy. As meteorites rapidly plunge through the atmosphere, tremendous friction with the air causes their exterior to heat up red-hot or even white-hot. The high speeds of the falling meteorites mean they have tremendous kinetic energy. This energy gets converted into heat through friction and air resistance. The heated meteorite exterior radiates light, creating the bright meteors and fireballs visible when meteorites impact earth’s atmosphere at high speeds.
Efficiency of Conversion
When kinetic energy is converted into heat through friction or other means, usually only a portion of the initial kinetic energy gets transformed into thermal energy. The percentage of kinetic energy that gets converted into heat depends on several factors:
Friction Coefficient
The friction coefficient of the surfaces in contact is a key determinant of efficiency. Materials with higher friction coefficients will convert more of the kinetic energy into heat. For example, rubber on road has a higher coefficient than steel on steel.
Contact Area
The greater the contact area between moving surfaces, the more kinetic energy will get converted into heat. Larger contact patches increase friction and heat generation.
Speed Differential
The larger the speed difference between two surfaces, the higher the heat generation. Kinetic energy increases exponentially with velocity, so faster speeds greatly improve efficiency.
Pressure and Weight
Higher pressure and weight on contact surfaces also increase heat efficiency. The greater the normal force, the more friction will occur during motion.
Optimizing these parameters allows the kinetic energy to heat conversion process to become more efficient. In ideal conditions, over 90% of kinetic energy can be converted into heat through friction.
Relationship to Thermodynamics
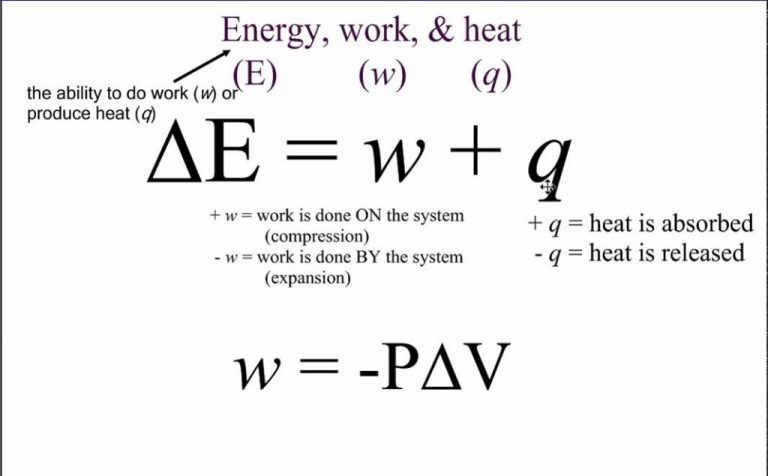
The conversion of kinetic energy into heat is closely related to the laws of thermodynamics. The first law of thermodynamics states that energy can neither be created nor destroyed in an isolated system. When kinetic energy is converted into heat, the total amount of energy remains the same. However, some of the energy is “lost” or becomes less usable in the process.
This leads to the second law of thermodynamics, which states that the entropy of an isolated system always increases over time. Entropy is a measure of disorder and randomness. When kinetic energy is converted into heat through friction, for example, the orderly motion of the objects is turned into the disordered motion of heat particles. This increases the entropy of the system.
The second law also states that it is impossible to convert heat completely back into useful work without some energy being lost. So when kinetic energy is converted into heat, it becomes more difficult to recover all of that initial kinetic energy. Some of the energy will be dissipated and spread out.
Understanding these thermodynamic principles helps explain why heat is generated when kinetic energy is converted through friction, as well as the limits on efficiency when trying to harness this kinetic energy. The entropy increase dictates that some energy will always be “lost” and turned into disordered heat when kinetic energy is converted.
Practical Applications
Kinetic energy is converted into heat in various practical applications through intentional design and engineering. Understanding how to maximize or minimize this conversion allows us to optimize systems for desired outcomes.
For example, brake systems in vehicles are designed to convert the kinetic energy of motion into heat through friction. Brake pads or shoes rub against a rotor or drum to slow the vehicle, and this friction generates significant heat. Engineers optimize brakes to dissipate heat quickly to avoid overheating. Ceramic compounds, drilled/slotted rotors, and brake cooling ducts help manage heat buildup.
On the flipside, engineers minimize unwanted heat generation in other applications. For example, high-speed aircraft and spacecraft require thermal protection systems or heat shields on the exterior to prevent excessive heating. As kinetic energy is converted to heat through atmospheric friction, the heat shield absorbs and dissipates the thermal energy to protect the craft and contents inside.
Understanding the relationship between kinetic energy and heat allows us to engineer solutions for converting, controlling, or preventing the heat transfer. Whether we want to maximize heating for braking systems or minimize heating for reduced drag and fuel efficiency, the conversion of motion into thermal energy is a key consideration in design and optimization across many fields.
Summary
As discussed throughout this article, kinetic energy and heat are closely connected through various mechanisms in science and nature. Kinetic energy, which is the energy of motion, can be converted into thermal energy, or heat, through friction, absorption, compression, and other processes.
The main points covered included:
- Kinetic energy increases with the speed of an object.
- Friction converts motion into heat as surfaces rub against each other.
- Absorption converts kinetic energy to heat as the energy is transferred to atoms and molecules.
- Compressing a gas leads to an increase in temperature as the kinetic energy is concentrated.
- Other mechanisms like eddy currents also enable the transformation from movement to heat.
Understanding the connection between kinetic energy and heat is important for thermodynamics and practical applications like brakes, turbines, and thermal management. This ability to convert between different forms of energy reveals the fundamentally interlinked nature of physics and the natural world.