Can Solar Energy Be Transformed Into Fuel?
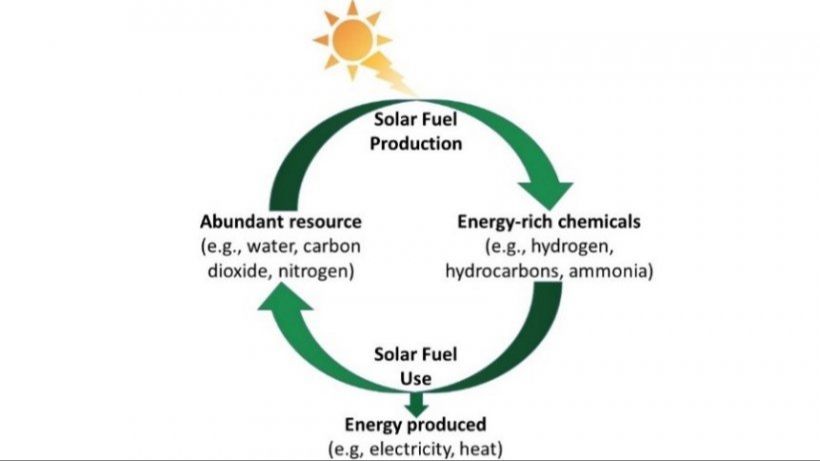
Solar energy is an abundant, renewable resource that has tremendous potential to meet the world’s energy needs in a sustainable way. The amount of solar energy that reaches the Earth’s surface in one hour is enough to meet the world’s annual energy demand (1). However, a key challenge is that solar energy is difficult to store and transport efficiently over long distances in the form of electricity. Transforming solar energy into a liquid or gaseous fuel that could be more easily stored and transported would be a major breakthrough (2). This would allow solar energy to be more fully harnessed as a mainstream energy source.
(1) https://www.seia.org/solar-industry-research-data
(2) https://www.marketwatch.com/guides/solar/solar-energy-statistics/
How Solar Energy is Currently Used
The two main uses of solar energy today are for generating electricity and for heating or cooling buildings. Solar photovoltaic (PV) panels convert sunlight directly into electricity using semiconducting materials. Arrays of solar PV panels are used in residential, commercial and utility-scale applications to provide electricity to homes, businesses or feed into the electric grid. According to the Solar Energy Industries Association, the amount of solar PV capacity in the U.S. has grown more than 24,000% since 2008, with over 3% of total electricity generation now coming from solar PV. The future growth prospects for solar PV are strong, with costs continuing to fall and energy policies supporting adoption.
Solar heating and cooling (SHC), also known as solar thermal, involves harnessing heat from the sun to provide hot water and space heating for residential and commercial buildings. Solar collectors, often mounted on rooftops, absorb and retain heat from the sun which can then heat water or interior space. Solar thermal is also used for solar cooling via absorption chillers. The SHC market has grown substantially in recent years, especially for heating swimming pools and domestic hot water which comprises over 90% of current installations. Solar thermal can provide over half the hot water needs for many buildings, and advancements in energy storage improvements may allow greater SHC applications in the future.
Methods to Convert Solar Energy to Fuel
There are several different approaches being researched to convert solar energy into fuel. Some of the main methods include:
Photoelectrochemical Conversion
Photoelectrochemical conversion uses solar energy to split water into hydrogen and oxygen. This process is also known as water splitting. Semiconductors like titanium dioxide are often used as photoelectrodes. When sunlight strikes the semiconductor it energizes electrons which then assist in breaking the chemical bonds in water to produce hydrogen gas which can be used as fuel (NREL).
Thermochemical Conversion
Thermochemical conversion relies on heat from concentrated solar power to drive chemical reactions that split water and carbon dioxide into hydrogen, carbon monoxide, and other compounds. The extreme heat allows the chemical bonds to break and reform into syngas and other fuel products (DOE).
Artificial Photosynthesis
Artificial photosynthesis aims to replicate the natural process of photosynthesis in plants. This involves using sunlight, water, and carbon dioxide to create carbohydrates and oxygen. Artificial systems use solar energy to convert water and CO2 into oxygen and chemical compounds like methanol which can be used as fuels (Wikipedia).
Photoelectrochemical Conversion
Photoelectrochemical (PEC) conversion uses semiconductor materials to directly convert sunlight into storable chemical fuels like hydrogen. When sunlight hits the semiconductor, it energizes electrons which can then be captured to split water molecules into hydrogen and oxygen. The hydrogen can then be used as a fuel source.
PEC cells use light-absorbing semiconductors like titanium dioxide as photoelectrodes. At one electrode, photons from sunlight energize electrons which are then transferred to the counter electrode through an external circuit. This electron flow converts water into oxygen and hydrogen gas. The electrode materials and electrolyte solution are critical components of PEC cells [1].
Key advantages of PEC fuel generation include the direct conversion of solar energy into fuels like hydrogen without the need for electric current. It also utilizes inexpensive and abundant semiconductor materials. However, challenges remain in improving the efficiency and stability of PEC electrode materials [2].
Thermochemical Conversion
Thermochemical conversion utilizes high temperature cycles driven by concentrated solar thermal energy to decompose water or carbon dioxide into hydrogen and oxygen or carbon monoxide and oxygen. The high temperatures, often over 1500°C, are attained through concentrating sunlight with mirrors onto a chemical reactor.
According to research from the National Renewable Energy Laboratory (NREL), thermochemical cycles have the potential to produce fuels from concentrated solar energy with much higher efficiency than by direct thermolysis 1. This is because the chemical reactants can be reproduced internally within the cycle. Thermochemical hydrogen production typically follows a two-step process. First, metal oxide is thermally reduced and oxygen is liberated, driven by concentrated solar heat. Then in the second step, the reduced metal oxide reacts with water to produce hydrogen.
There are many possible thermochemical cycles but the leading candidates for large scale solar fuel production via water splitting are ferrite cycles, ceria cycles, and zinc oxide cycles 2. These have been proven at demonstration scale but still face challenges with efficiency, durability, and costs before wide scale commercial deployment.
Artificial Photosynthesis
Artificial photosynthesis aims to mimic the natural process that occurs in plants by using nanomaterials and catalysts to convert sunlight, water, and carbon dioxide into fuel. This process uses solar energy to split water into hydrogen and oxygen. The hydrogen can then be combined with carbon dioxide to produce hydrocarbon fuels.
Nanomaterials like titanium dioxide nanoparticles are used to absorb sunlight and separate the charges needed for water splitting, similar to how chlorophyll captures light in plants. Oxygen evolution catalysts like cobalt oxide are used to facilitate the oxygen production reaction.
Meanwhile, the hydrogen ions interact with a hydrogen evolution catalyst like platinum to make hydrogen gas. The CO2 is then converted into fuels like methanol by reducing the carbon and adding hydrogen.
The advantage of artificial photosynthesis is that it provides a renewable way to make storable, carbon-neutral fuels using only sunlight, water, and CO2 as inputs. However, current systems only convert a small percentage of solar energy into fuel. More efficient nanomaterials and catalysts are needed to improve the conversion ratios before artificial photosynthesis can be commercially viable. If achieved, this technology could provide sustainable transportation fuels from an abundant renewable resource.
Recent breakthroughs like self-assembling co-catalysts and new photocathode materials demonstrate progress towards making artificial photosynthesis systems more efficient and scalable. With continued research, solar fuels from artificial photosynthesis may eventually become a major contributor to the renewable energy economy. (1)
Challenges to Overcome
There are several key challenges that need to be overcome before solar fuels can become a widespread, commercially viable technology (DOE Explains…Solar Fuels). These include:
- High costs – Current methods for producing solar fuels remain expensive compared to fossil fuels. More research is needed to find lower cost materials and improve efficiencies to reduce overall costs.
- Low efficiencies – Most devices that convert solar energy to fuels have relatively low sunlight-to-fuel efficiencies, often less than 10%. Higher efficiencies are needed to make the process commercially viable.
- Difficulty scaling up – Many solar fuel technologies work well in the lab but face challenges when scaled up to industrial levels. New materials and designs are needed.
According to the Department of Energy, the cost of solar fuels needs to be reduced to around $3 per gallon of gasoline equivalent or less to be economically competitive. Reaching this target will require new, low-cost materials and system architectures that operate with high efficiency and stability when scaled up (DOE Explains…Solar Fuels). Overcoming these challenges is an active area of research.
Recent Breakthroughs
There have been some promising recent advances in solar fuel research and development. In 2021, researchers at the University of Cambridge developed an artificial leaf that uses sunlight to convert water and carbon dioxide into oxygen and synthesis gas, which can be used to produce fuels like hydrogen, methanol and gasoline (DOE Explains…Solar Fuels). The leaf is made of a semiconductor that absorbs sunlight and catalyzes the conversion reactions. In lab tests, it was able to convert 10% of the solar energy into fuel, which is a significant step towards efficient solar fuel production.
Another recent breakthrough came from researchers at ETH Zurich, who engineered bacteria that can convert sunlight, water and air into a compound called isobutene. Isobutene can be processed into gasoline, jet fuel and other hydrocarbon fuels. The bacteria use photosynthesis to convert carbon dioxide into isobutene gas. While the conversion efficiency is currently low, this research demonstrates the possibility of biologically-based solar fuel production (Solar fuels: the clean alternative to fossil fuels).
Future Outlook
The future of solar energy looks very promising, with projections for major cost reductions and efficiency gains that could enable solar power to make a significant global impact.1 In particular, continued improvements in photovoltaic technology and energy storage are expected to dramatically lower costs over the next decade.
One projection estimates that by 2024, solar panel costs could decrease by 60% compared to today’s prices, while storage costs could fall by 50-70%.2 At the same time, average solar cell efficiencies are predicted to increase from 20% now to over 25% in the near future.3
With these developments, solar power is poised to become cost competitive with fossil fuels across the globe. This could enable widespread solar adoption and allow solar to provide over 20% of global electricity by 2030.1 Some even see potential for solar to make up 50% or more of the world’s energy mix by mid-century.3 This would greatly reduce greenhouse gas emissions and dependence on non-renewable energy sources.
Conclusion
The ability to convert solar energy directly into fuel would be a major breakthrough that could significantly reduce reliance on fossil fuels. Solar energy is an abundant renewable resource and transforming even a fraction of it into usable fuel could help curb greenhouse gas emissions and mitigate climate change.
There are several promising methods for converting solar energy to fuel, but more research and development is needed to improve efficiency and bring costs down. Recent breakthroughs in materials science, nanotechnology, and artificial photosynthesis provide hope that economical solar fuel generation may be achievable in the coming decades.
With sufficient investment in R&D and supportive government policies, solar fuel technologies could become a major part of the global energy mix. The potential benefits make this an important area for ongoing research. Further innovations will be key to unlocking the full potential of solar energy as a renewable fuel source.