Can Energy Be From One Form To Another Bill Nye?
Energy is the ability to do work or produce heat. It exists in various forms that can be grouped into two main categories: potential energy and kinetic energy. Potential energy is stored energy based on an object’s position or arrangement. For example, a ball held at a height above the ground has gravitational potential energy. Kinetic energy is energy of motion. A ball rolling down a hill has kinetic energy.
There are several common types of energy:
- Mechanical energy is the total potential and kinetic energy in a system.
- Chemical energy is energy stored in the bonds between atoms and molecules. It is released in chemical reactions.
- Electrical energy is energy derived from electric charges or fields.
- Nuclear energy comes from reactions within atomic nuclei.
- Thermal energy is the internal energy of substances from the motion of atoms and molecules.
- Radiant energy is electromagnetic energy that travels in transverse waves, like visible light.
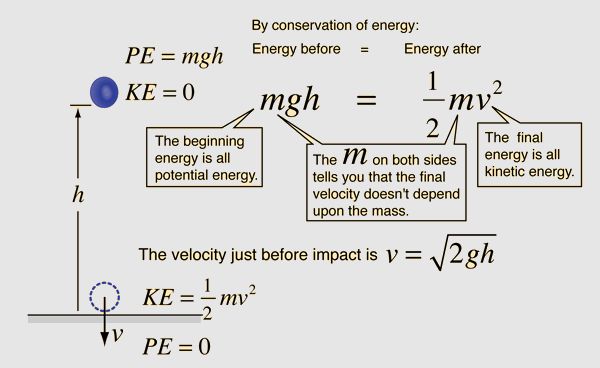
According to the law of conservation of energy, energy can change forms but is never created or destroyed. For example, when a ball falls, its potential energy transforms into kinetic energy. Understanding how energy transforms between forms is key to harnessing it for human use.
Potential Energy
Potential energy is the energy stored in a system or object that has the potential to do work. Some common examples of potential energy include:
- Gravitational potential energy – This is energy that an object possesses due to its height above the ground. For example, a book sitting on a high shelf has more gravitational potential energy than a book sitting on the floor.
- Elastic potential energy – The energy stored in elastic materials that are deformed or stretched. For example, a compressed spring or stretched rubber band has elastic potential energy.
- Chemical potential energy – The energy stored in the bonds between atoms and molecules. This energy gets released when chemical bonds are broken during a chemical reaction.
- Nuclear potential energy – The energy that binds together the protons and neutrons in the nucleus of an atom.
The key thing about potential energy is that it is stored energy that has the potential to be released and do work. An object must have potential in order for it to have potential energy. This potential can come from the object’s position, composition, or state. As soon as the potential is released, the potential energy gets converted into kinetic energy or motion.
Kinetic Energy
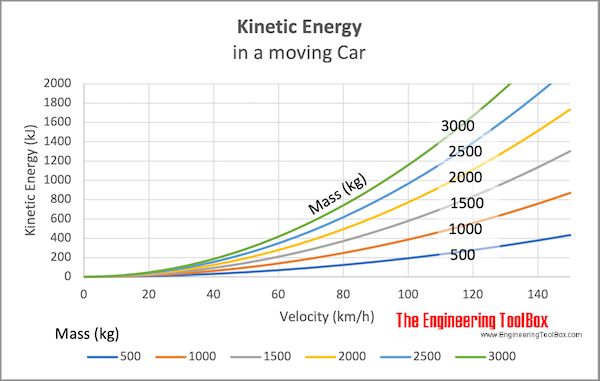
Kinetic energy is energy associated with motion. An object that is in motion, like a rolling ball or a moving car, has kinetic energy. The amount of kinetic energy depends on the mass and speed of the moving object. The more massive the object and the faster it moves, the more kinetic energy it has.
Kinetic energy can be described by the equation:
KE = 1/2 * mass * velocity^2
Where KE is kinetic energy, mass is the object’s mass, and velocity is the speed of the object. This shows that as velocity increases, kinetic energy increases exponentially. For example, doubling the velocity quadruples the kinetic energy.
A moving object’s kinetic energy comes from its momentum. So kinetic energy demonstrates the work needed to accelerate a mass from rest to its stated velocity. Many examples in everyday life involve converting potential energy into kinetic energy, like a ball rolling down a hill or a roller coaster moving down its tracks.
Mechanical Energy
Mechanical energy is the energy associated with the motion and position of objects. There are two types of mechanical energy:
Kinetic Energy
Kinetic energy is the energy of motion. Moving objects, like cars driving down the road or kids playing on a swing set, have kinetic energy. The faster something moves, the more kinetic energy it has. Kinetic energy can be transferred between objects during collisions.
Potential Energy
Potential energy is stored energy based on an object’s position or shape. A ball held at the top of a ramp has potential energy because of its position at the top. When released, this potential energy gets converted into kinetic energy as the ball rolls down. Other examples include a compressed spring and objects at high altitudes.
Mechanical energy can easily change form between kinetic and potential energy. A skateboarder at the top of a ramp has potential energy that turns into kinetic energy as she speeds down. The kinetic energy turns back into potential energy as she reaches the top of the next ramp.
Chemical Energy
Chemical energy is energy stored in the bonds between atoms and molecules. It is the energy that holds these particles together. We experience chemical energy being converted into other forms of energy during chemical reactions.
For example, the food we eat contains chemical energy that our bodies convert into thermal and mechanical energy through metabolism. Gasoline and other fuels also contain chemical energy that is released as heat and kinetic energy when the fuel undergoes combustion in a car engine.
Chemical energy can involve breaking and forming of chemical bonds. An exergonic reaction like combustion is when bonds are broken and chemical energy is converted into thermal energy. An endergonic reaction is when bonds are formed, absorbing thermal energy and storing it as chemical energy.
Batteries are another example of chemical energy being converted into electrical energy through redox reactions. The potential chemical energy stored in the battery materials is released as electricity.
Electrical Energy
Electrical energy is associated with the motion of electrons, or the particles that carry a negative electrical charge. Atoms consist of a nucleus surrounded by orbiting electrons. Electrical energy refers to the ability of electrons to do work as they flow from one atom to another.
Electrons can move freely between atoms in materials called conductors, like metals. In insulators, like rubber or plastic, electrons are tightly bound to their atoms and cannot flow freely. When electrons move between atoms, an electric current is created. The force driving this electron flow is called voltage. Voltage causes electrons to flow through a conductor, like a copper wire, releasing energy.
For example, a battery contains stored chemical energy that is converted into electrical energy when the battery is connected in a circuit. The voltage provided by the battery applies a force that causes electrons to move through the wires and components. This flow of electrons is the electric current that allows the circuit to do work, like light a lightbulb, power a motor, or run a computer.
The movement of electric charges creates a magnetic field around a conductor. Changing magnetic fields produce electromagnetic waves. This is how energy is transferred in electrical and electronic devices to accomplish useful tasks.
Nuclear Energy
Nuclear energy comes from the splitting of atoms in a process called nuclear fission or the fusion of atoms in a process called nuclear fusion. In nuclear fission, the nucleus of a heavy atom like uranium or plutonium is split into smaller nuclei, releasing a tremendous amount of energy in the process. In nuclear fusion, light nuclei are fused together to form heavier nuclei, also releasing massive amounts of energy.
The reason nuclear fission and fusion release so much energy is because of the strong nuclear force that binds protons and neutrons together in the nucleus. When the nucleus is split or fused, the reconfiguration of the nuclear force leads to a release of energy according to Einstein’s equation E=mc^2. This enormous amount of energy packed into the tiny nucleus of an atom is what makes nuclear power so appealing as an energy source.
Nuclear fission is used in nuclear power plants to produce electricity. The energy released by splitting uranium or plutonium atoms heats water to produce steam, which spins a turbine to generate electricity. Nuclear fusion is more difficult to achieve in a controlled manner for power generation, but it promises even greater amounts of energy from small amounts of fuel if it can be harnessed.
Nuclear energy has advantages as an electricity source, including the ability to produce large amounts of power from a small amount of fuel. However, it also carries risks like radiation, nuclear accidents, and issues around nuclear waste storage. Overall, nuclear power demonstrates how the microscopic world of atoms and nuclei contains tremendous energy that we are still learning how to utilize safely.
Thermal Energy
Thermal energy, also known as heat energy, is the internal energy present in all matter. It arises from the motion of atoms and molecules inside substances. The faster these particles move, the more thermal energy they possess.
Thermal energy can be transferred from one object or system to another. This transfer happens in three main ways – conduction, convection, and radiation.
Some examples of thermal energy in everyday life include:
- The warmth from the sun that we feel on our skin
- The heat that cooking food on a stove generates
- The hot air that blows from a hair dryer
- The warmth that transfers from a hot drink to your hands
Thermal energy always flows spontaneously from objects at higher temperatures to objects at lower temperatures until they reach equilibrium. This natural flow of heat is the driving force behind many essential technologies and systems that provide heating, cooling, electricity generation, and more.
Radiant Energy
Radiant energy is energy that travels in the form of electromagnetic waves or photons, including visible light, radio waves, gamma rays, and others. It can also be described as energy that is radiated or spread out from a source. Unlike other forms like thermal or mechanical energy that rely on particle movement, radiant energy relies only on electromagnetic radiation propagating through space.
The most familiar example of radiant energy is visible light from sources like the Sun, light bulbs, and flames. Light is a form of radiant energy that we can detect with our eyes. Other forms like ultraviolet light, x-rays, and radio waves are not visible to us but are still examples of radiant energy. Radiant energy is produced any time an atom or molecule makes a transition from a higher energy state to a lower energy state and releases a photon in the process.
Radiant energy can travel over long distances and is capable of transferring energy from one location to another without relying on particle movement. It does not require a medium like air or water to travel through. This allows sunlight to travel through the vacuum of space to reach Earth. One unique property of radiant energy is that it exhibits both wave-like and particle-like behavior.
Law of Conservation of Energy
The law of conservation of energy states that energy can neither be created nor destroyed, it can only be transformed from one form to another. This means the total energy in an isolated system always remains constant. For example, when a ball falls, its potential energy gets converted to kinetic energy. The total amount of energy before and after the fall remains the same.
Energy transformations happen all the time around us. Chemical energy in food gets converted to thermal and mechanical energy in our bodies. Electrical energy gets converted to light and heat energy in a light bulb. In an ecological system, radiant energy from the sun is transformed and transferred between different organisms. The law of conservation of energy governs all these changes and states that the total amount of energy remains fixed.
While energy can change form, it cannot be created from nothing or destroyed into nothing. The amount of energy present before and after a transformation will always be equal. This principle applies across all fields of science and is one of the fundamental laws governing our universe. Understanding the law of conservation of energy allows us to track how energy flows and changes within any system.