Why Is Kinetic Energy Important To Chemists?
Kinetic energy is the energy associated with motion. It is directly proportional to the mass of an object and the square of its velocity. In chemistry, kinetic energy plays a critical role in chemical reactions, reaction rates, diffusion, effusion, phase changes, thermodynamics, catalysis, and spectroscopy.
The kinetic energy of molecules is a key concept in the kinetic molecular theory, which aims to explain the behavior of gases. According to this theory, gases consist of molecules in constant random motion. The kinetic energy of these moving molecules is directly related to the temperature of the gas. As temperature increases, the average kinetic energy of the gas molecules also increases.
Kinetic energy is vitally important in driving many essential chemical processes. Understanding how kinetic energy affects collisions between molecules, diffusion rates, reaction rates, phase changes, and more provides chemists with invaluable insights into chemical systems on the molecular level.
Kinetic Molecular Theory
Kinetic molecular theory seeks to explain the behavior of gases by describing the motions and collisions of the constituent gas molecules. According to this theory, gas molecules are constantly moving and colliding with each other and the walls of their container. The kinetic energy of a gas molecule is directly related to its velocity and temperature.
As temperature increases, gas molecules move faster on average and therefore have higher average kinetic energy. This increased molecular motion corresponds to an increase in pressure as the molecules collide more frequently with the walls of their container. Conversely, decreasing temperature causes gas molecules to move more slowly and carry less kinetic energy, lowering the pressure as fewer collisions occur.
The relationship between molecular kinetic energy and temperature is very important in chemistry. Many chemical reactions accelerate at higher temperatures because the reactants have more kinetic energy to overcome the activation barrier. Kinetic molecular theory provides a mechanism to explain this observation, as higher temperatures impart more kinetic energy to the reactant molecules.
Kinetic theory also helps explain diffusion and effusion rates. At higher temperatures, gas molecules are moving faster so they diffuse and effuse more rapidly. The principles of kinetic molecular theory thus tie together the microscopic details of molecular motion with observable bulk properties of gases.
Chemical Reactions
Kinetic energy plays a critical role in initiating chemical reactions. In order for a chemical reaction to occur, the reactant molecules must collide with sufficient energy to break the bonds in the reactants and form new bonds to create the products. This minimum required energy is known as the activation energy.
Without enough kinetic energy, the reactant molecules may collide but will simply bounce off each other. The kinetic energy enables them to distort their structures and bonds temporarily to allow new bonds to form. Kinetic energy helps overcome the activation barrier so the chemical reaction can proceed.
Increasing the temperature increases the average kinetic energy of the molecules. With more kinetic energy, a greater fraction of collisions will have enough energy to exceed the activation barrier. This is why most chemical reactions proceed faster at higher temperatures – the kinetics are accelerated.
Catalysts work by providing an alternative reaction pathway with a lower activation energy. Less kinetic energy is required for the catalyzed reaction to occur. By reducing the required activation energy, a catalyst increases the rate of a chemical reaction by enabling more molecular collisions to result in reaction.
Understanding how kinetic energy affects chemical reactions allows chemists to control the speed and yield of reactions. Chemists can manipulate kinetic energy by changing temperature, concentration, surface area, or using catalysts to optimize chemical processes.
Reaction Rates
The rate of a chemical reaction depends on the frequency and energy of collisions between reactant molecules. According to collision theory, molecules must collide with sufficient energy and proper orientation for a reaction to occur. The minimum energy required for a reaction is called the activation energy.
Increasing the temperature increases the average kinetic energy of the molecules. With more kinetic energy, a larger fraction of molecular collisions have energy greater than the activation energy. This increases the likelihood that collisions between reactant molecules will result in a chemical reaction. Therefore, higher temperatures usually correspond to faster reaction rates.
The kinetic energy of molecules is directly proportional to temperature. Doubling the temperature increases the average kinetic energy of the molecules by a factor of two. This exponential relationship between temperature and kinetic energy is the reason that small changes in temperature can produce large changes in reaction rates.
In summary, the kinetic energy of molecules determines the fraction of collisions with enough energy to react. Higher temperatures increase kinetic energy which speeds up reaction rates based on collision theory.
Diffusion and Effusion
Diffusion and effusion rates of gases are directly affected by the kinetic energy of the gas molecules. Diffusion refers to the intermingling of molecules as they move from an area of higher concentration to an area of lower concentration. Effusion refers to the escape of molecules through a tiny opening or porous barrier. In both cases, the speed and kinetic energy of the individual gas molecules determine how quickly they can spread out and pass through openings.
Higher kinetic energy means the molecules are moving faster on average. Therefore, gases with higher temperatures, and thus higher average kinetic energies, will diffuse and effuse more rapidly. The diffusion rate or effusion rate is proportional to the square root of the absolute temperature of the gas. This relationship is described quantitatively by Graham’s law of effusion and Fick’s law of diffusion.
Additionally, lighter gas molecules, with fewer atoms, have less mass and thus naturally move faster than heavier gas molecules, for the same kinetic energy. So at the same temperature, lighter gases like helium diffuse and effuse faster than heavier gases like xenon or radon. The precise mathematical relationship comparing diffusion/effusion rates and molecular masses is provided by the Graham’s law and Fick’s law equations.
Understanding how kinetic energy affects diffusion and effusion allows chemists to predict gas behavior and model processes like respiratory gas exchange in the lungs, industrial separation of gases, pharmaceutical drug delivery, and more. The principles of diffusion and effusion are foundational concepts in chemistry and physics.
Phase Changes
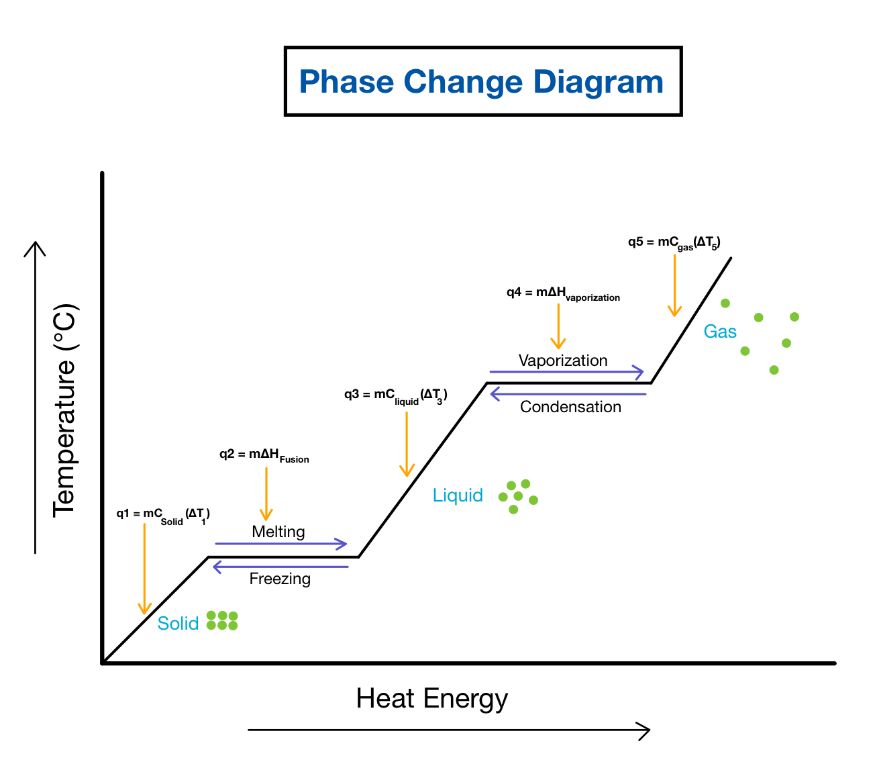
Kinetic energy plays a critical role in phase changes like melting, boiling, evaporation, freezing, deposition, and condensation. These phase transitions occur when molecules gain or lose enough kinetic energy to overcome the intermolecular forces holding them together in a particular state of matter.
For example, when a solid is heated, the molecules vibrate more rapidly and their kinetic energy increases. At the melting point, the molecules have gained enough kinetic energy to overcome the forces holding them in place in the solid lattice. This allows the molecules to break free and move about more freely in the liquid phase.
Similarly, as a liquid is heated, the kinetic energy continues to increase until the molecules have enough energy to completely break free of each other and enter the gaseous phase at the boiling point. The reverse processes occur during freezing, condensation, and deposition as molecules lose kinetic energy.
The amount of kinetic energy required for a phase change depends on the strength of the intermolecular forces. Stronger forces require more kinetic energy to overcome, which is why substances with stronger intermolecular forces have higher melting and boiling points.
Kinetic energy is the driving factor that enables molecules to transition between solid, liquid, and vapor states. Understanding kinetic energy is key for chemists studying thermodynamics, material properties, and chemical processes involving phase changes.
Thermodynamics
Kinetic energy is intimately related to thermodynamic properties like internal energy, enthalpy, and entropy. The internal energy of a system is the sum of all kinetic and potential energies of the molecules within it. Since kinetic energy arises from molecular motion, a higher internal energy correlates with higher average kinetic energy of the molecules.
Enthalpy, or heat content, is another important thermodynamic property related to kinetic energy. The change in enthalpy (ΔH) of a chemical reaction is equal to the heat absorbed or released at constant pressure. Exothermic reactions that release heat have lower enthalpy of products than reactants. This is because some kinetic energy is converted into potential energy during bond making and breaking. The lost kinetic energy emerges as heat.
Entropy, which measures molecular randomness and disorder, also relates to kinetic energy. Higher kinetic energy allows more ways a system can be arranged (higher positions/momenta for molecules), increasing entropy. Temperature, which reflects average kinetic energy, is directly proportional to entropy. An increase in kinetic energy of molecules leads to higher entropy.
Overall, kinetic energy is a fundamental driver of thermodynamic properties and processes. Chemists apply thermodynamics principles to predict if reactions will occur spontaneously based on enthalpy and entropy changes related to kinetic energy transfers and transformations.
Catalysts
Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. They work by providing an alternative reaction pathway that has a lower activation energy barrier. The activation energy is the minimum amount of energy that reactants must possess before they can undergo a chemical reaction.
Catalysts orient reactants in ways that make it easier for their bonds to break or form. They also introduce additional vibrational and kinetic energy to the reactants. This combination of orientation and added kinetic energy enables more molecular collisions between reactants to result in successful reactions. With a catalyst, more reactant molecules have enough energy at a given temperature to get over the lowered activation barrier and convert to products.
For example, enzymes are catalytic proteins that accelerate the rate of biochemical reactions in cells by factors of a million or more. Their complex shapes provide complementary binding sites for substrate molecules. This positions the substrates in an optimal orientation for the reaction. The enzyme also flexes and vibrates, which transfers kinetic energy to the bound substrates. Altogether, this catalytic effect produces enormous rate enhancements for vital biochemical processes.
In summary, catalysts work by lowering the activation energy needed for a reaction to occur. They achieve this through proper orientation of reactants and transferring kinetic energy to them. This allows more successful reactions to take place at a given temperature, greatly accelerating the rate of reaction.
Spectroscopy
Kinetic energy plays an important role in spectroscopy, which is the study of how matter interacts with electromagnetic radiation. Spectroscopic techniques allow chemists to probe the energy levels of molecules by measuring how they absorb or emit light.
When molecules absorb light, they undergo transitions between different rotational and vibrational energy levels. The frequencies of light absorbed correspond to the energy spacing between these levels. The more kinetic energy a molecule has, the more closely spaced its rotational and vibrational levels become. This means molecules with more kinetic energy will absorb light at higher frequencies.
Likewise, when molecules emit light, the frequencies observed are determined by the energy spacing between levels. More kinetically energetic molecules rotate and vibrate more rapidly, leading to emissions at higher frequencies. The spectral lines are also broader for molecules with greater kinetic energy due to uncertainty in the exact energies of the transitions.
By analyzing the frequencies and bandwidths of spectral lines, chemists can determine the distribution of kinetic energies within a molecular sample. This provides valuable insights into molecular structure, bonding, and intermolecular interactions. Understanding how kinetic energy affects spectra is essential for interpreting and applying spectroscopic measurements in chemistry.
Conclusion
Kinetic energy plays a vital role across many areas of chemistry. At its core, kinetic energy drives the motion and interactions of molecules and atoms. This manifests in countless chemical processes from the foundational behavior described by the kinetic molecular theory to complex biological interactions and technological applications.
The kinetic energy of molecules and atoms governs reaction rates, allowing chemists to predict and control the speed of chemical changes. It facilitates important processes like diffusion and effusion which are critical for biological systems. Phase changes between solids, liquids and gases are also dictated by kinetic energy levels.
In thermodynamics, kinetic energy is a key component for describing the energy transfers within and between systems. The activation energy provided by kinetic energy allows reactions to occur. Kinetic energy enables catalysts to accelerate chemical reactions by reducing this activation barrier.
Spectroscopic techniques like infrared spectroscopy rely on kinetic energy transitions between molecular energy levels. Overall, kinetic energy provides a fundamental bridge between the microscopic properties of atoms and molecules and the macroscopic observations made in the laboratory and nature.
By studying the kinetic energy and motion of chemical species, chemists gain profound insight into properties, behaviors and processes at scales spanning the subatomic to the observable. An understanding of kinetic energy is invaluable across all branches of chemistry.