Why Is A Solar Cell Called A Pv Cell?
Solar cells, also known as photovoltaic (PV) cells, are devices that convert sunlight into electricity. They are made up of one or more semiconductor materials, most commonly silicon, that absorb photons from sunlight and release electrons. The flow of these electrons generates an electric current that can be captured and used as electricity. This process of converting light (photons) to electricity (voltage) is called the photovoltaic effect.
In the simplest terms, solar cells directly transform energy from the sun into usable electricity that powers our homes, businesses, and grid. Solar energy is a renewable and sustainable resource that does not create any emissions or pollution. Solar power provides a clean alternative to burning fossil fuels for energy. Understanding the photovoltaic process that enables solar cells to generate electricity is key to harnessing this abundant energy source.
History of Solar Cells
The discovery of the photovoltaic effect dates back to the 19th century. In 1839, French physicist Edmond Becquerel first observed the photovoltaic effect while experimenting with metal electrodes in electrolyte solutions. Becquerel noticed that certain materials would produce small amounts of electric current when exposed to light.
However, it took over a century for the first silicon solar cell to be developed. In 1954, researchers at Bell Laboratories in the United States created the first practical silicon photovoltaic cell with a power conversion efficiency of around 6%. This pioneering solar cell paved the way for the photovoltaic technology we know and use today.
What is a Photovoltaic Effect?
The photovoltaic effect is the basic physical process that allows solar cells to produce electricity. When sunlight hits the solar cell, the photons (particles of light) strike the solar cell material, usually silicon. If the photons have enough energy, they will kick electrons loose from the atoms of the solar cell material, creating a flow of electricity.
Specifically, here’s how it works:
- Photons from sunlight hit the solar cell material and get absorbed.
- If the photon has enough energy, it will dislodge an electron from an atom of the cell’s semiconductor material.
- The freed electron then contributes to the flow of an electric current in an external circuit.
This process of converting light (photons) to electricity (voltage and current flow) is called the photovoltaic effect. It was first discovered in 1839 by French physicist Edmond Becquerel. The term “photovoltaic” comes from the Greek word for light, “phos”, and “voltaic”, referring to electricity.
PV: Photovoltaic
The “PV” in the term “PV cell” stands for “photovoltaic.” This refers to the photovoltaic effect, which is the process that enables solar cells to convert sunlight into electricity.
The photovoltaic effect occurs when photons, or particles of light, strike the surface of certain materials like silicon and generate electricity. Specifically, the photons knock electrons loose from their atoms, allowing the electrons to flow through the material and produce an electric current. This conversion of light (photons) to electricity (voltage) is known as the photovoltaic effect.
When sunlight hits the surface of a solar cell, which is made of a photovoltaic material like silicon, the photovoltaic effect generates voltage between two layers in the cell. The more sunlight that hits the solar cell, the more electricity that is produced through the photovoltaic effect.
In summary, the term “photovoltaic” refers to the ability of solar cells to directly convert sunlight into usable electrical energy through the photovoltaic effect. This is why solar cells are called PV cells.
Components of a Solar Cell
Solar cells are made of various semiconductor materials that exhibit the photovoltaic effect. The most common material used is silicon, which can be treated with various doping agents to form either a p-type or n-type semiconductor. The basic components of a solar cell are:
- p-type semiconductor layer – Usually made of silicon doped with boron, which adds “holes” for positive charge carriers.
- n-type semiconductor layer – Made of silicon doped with phosphorus, which adds extra electrons for negative charge carriers.
- Electrical contacts – Metal contacts are attached to the top and bottom of the sandwich of p-type and n-type silicon to collect the current.
When sunlight hits the solar cell, photons with enough energy knock electrons loose in both the p-type and n-type silicon layers. The built-in electric field generated at the junction between the two layers causes these electrons to flow in one direction, creating an electrical current. The electrical contacts collect this current for external use.
How Solar Cells Create Electricity
Solar cells generate electricity through the photovoltaic effect. Here is a step-by-step explanation of how this process works in a solar cell:
Solar cells are made of a special material called a semiconductor. Silicon is the most common semiconductor used. It has electrical properties between those of a conductor, like copper, and an insulator, like rubber. Silicon serves as the base material for solar cells.
When silicon is exposed to light, electromagnetic radiation from the sunlight dislodges electrons from the atoms in the semiconductor, allowing those electrons to flow through the material. This makes silicon a good conductor of electricity.
The key to generating electricity is to organize the electrons liberated by light absorption. This is done by creating an electric field within the cell that forces electrons freed by light in one direction, and positive charged holes the opposite way.
A solar cell contains a p-n junction made of two oppositely doped layers of silicon – a p-type with positive charge carriers and an n-type with negative charge carriers. At the point where these two layers meet, free electrons on the n-side diffuse across the junction to the p-side. This movement of charge carriers forms the electric field.
When sunlight enters the solar cell, photons strike the electrons in the p-n junction and excite them to a higher state of energy. This enables them to act as charge carriers and cross the junction. Sunlight creates an electrical imbalance across the pn junction, with electrons accumulating on the n-side and holes on the p-side. This separation of charge creates a voltage potential like a battery.
By connecting the positive and negative sides of the solar cell to an external load, electricity can flow through wires to power devices. And voilà, the photovoltaic effect converts sunlight into electrical energy!
Solar Cell Efficiency
In lab conditions, researchers have achieved impressive solar cell efficiency rates of over 45%. However, translating lab successes into real-world applications comes with many challenges. Factors like manufacturing defects, temperature fluctuations, and low light conditions can substantially reduce efficiency. Commercial grade solar panels typically operate between 15-20% efficiency. While lower than lab benchmarks, ongoing advancements in materials science and panel optimization continue to push commercial efficiency rates higher.
Improving real-world solar efficiency often boils down to reducing energy loss. Maximizing light absorption, minimizing manufacturing defects, and optimizing energy transfer pathways all play key roles. Additionally, newer multi-junction solar cells can capture a wider range of light frequencies – leading to higher efficiency potential. With global R&D and investments growing each year, solar technology is positioned to keep closing the gap between peak lab performance and commercial grade efficiency.
Applications of Solar Cells
Solar cells have many practical applications in our everyday lives. Some of the most common applications of solar cells and solar power include:
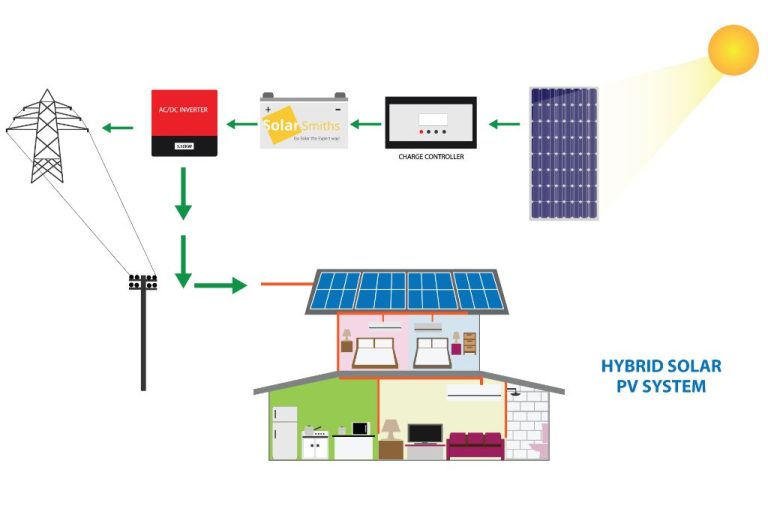
- Solar panels – Arrays of solar cells are used in solar panels to convert sunlight into electricity. Solar panels can range from small rooftop systems on homes and businesses to large utility-scale solar farms providing electricity to the grid.
- Calculators – Small solar cells provide power for many calculators, allowing them to function without batteries.
- Satellites – Solar cells are used to power satellites and spacecraft, allowing them to operate for years in space without fuel.
- Road signs/street lighting – Solar cells can power road signs, street lights, and other municipal infrastructure.
- Consumer electronics – Small solar panels can provide backup power or recharging for items like watches, radios, lanterns, and more.
- Cars/boats – Solar cells are being integrated into some electric vehicles to extend driving range and power boats or RVs off-grid.
Solar cell technology continues to advance and become more efficient and affordable. As a result, we will likely see solar cells integrated into even more aspects of our lives in the future.
Future of Solar Cell Technology
The future looks bright for solar cell technology. Researchers are constantly working to improve solar cell designs and materials to increase efficiency and lower costs. Some emerging innovations in solar cell technology include:
Perovskite solar cells – These new solar cells are made of perovskite materials that can be printed cheaply, similar to printing ink. Perovskites have achieved remarkable efficiency improvements in just a few years of research. They have potential as a flexible, lightweight and highly efficient solar material.
Quantum dot solar cells – Quantum dots are tiny nanoparticles that can absorb light and generate more than one electron per photon. This improves solar cell efficiency compared to traditional cells. Quantum dot solar cells are still in early stages of research but offer promising efficiency improvements.
Organic photovoltaics (OPV) – OPV solar cells use conductive organic polymers that can be dissolved and printed onto flexible substrates. While their efficiency is lower than traditional cells, OPV offers exciting possibilities like solar textiles or paint-on solar films.
Solar cell nanomaterials – Various nanomaterials like carbon nanotubes, graphene and nanowires are being researched to improve solar cell efficiency, save material costs and reduce weight. Nanomaterials can potentially enhance electrical conductivity and light absorption.
Space applications – Lightweight, flexible solar cells are enabling increased use in space, from powering satellites and spacecraft to future applications like off-planet manufacturing. The low-gravity environment enables new solar array designs.
With continued research and investment driven by the growing demand for renewable energy, solar cell technology has an exciting future ahead. We can expect to see solar cells become increasingly efficient, affordable and versatile.
Conclusion
In conclusion, solar cells are an important source of renewable energy that utilize the photovoltaic effect to convert sunlight directly into electricity. The key components of a solar cell include a p-n junction semiconductor, metal contacts, anti-reflective coating, and encapsulant. When sunlight hits the solar cell, photons with enough energy are absorbed and knock electrons loose in the semiconductor, creating electron-hole pairs. The built-in electric field of the p-n junction causes these electrons to flow in one direction, producing an electric current. While solar cells start with relatively low efficiency, research is ongoing to increase their efficiency and make solar energy more cost competitive with fossil fuels.
Solar cells have many applications and are used in everything from small consumer electronics to residential rooftop panels to large utility-scale solar farms. As solar technology continues to advance and costs decrease, solar electricity will play an expanding role in our energy future. The photovoltaic effect provides a direct way to harness the enormous power of the sun for human use. Solar cells are an essential technology for shifting to renewable energy sources and building a more sustainable world.