Why Does Light Change Materials?
Light is a form of electromagnetic radiation that allows us to see objects around us. Materials are substances that objects are made of, like wood, metal, plastic, etc. When light interacts with different materials, it can cause changes in the molecular structure and chemical properties of those materials.
There are several ways that light can alter materials. The photons or particles that make up light can be absorbed by atoms and molecules, transferring energy to them. This extra energy can break chemical bonds or induce new chemical reactions, modifying the material. The wavelength and intensity of the light affects how much it interacts with and transforms various materials.
In this article, we will explore some of the key examples of how light exposure leads to changes in materials. From photochromic sunglasses to sunburned skin, light’s ability to alter materials has many important applications and biological effects.
Photochromic Materials
Photochromic materials are substances that change color when exposed to sunlight or ultraviolet light. This is a reversible process, meaning the material will revert back to its original color once the light source is removed. Some common examples of photochromic materials include:
- Photochromic lenses used in eyeglasses that darken when exposed to UV light.
- Photochromic dyes used in fabrics and clothing that change color in the sun.
- Photochromic inks used in novelty items like mugs and t-shirts that change color when left in the sun.
The color change occurs due to a chemical reaction triggered by the UV light. Most photochromic materials contain silver halide crystals that change structure and absorb light differently when exposed to UV radiation. This absorbs certain wavelengths of light and reflects others, resulting in a color change. The process happens quickly, within seconds or minutes of UV exposure. Once the UV light source is removed, the silver halide crystals revert to their original formation and the material regains its original color.
The reversible color change makes photochromic materials popular for items like sunglasses and self-tanning products. The tint helps protect eyes from harsh sunlight while outdoors, then fades back to a clear lens when back inside. Photochromic dyes in sunscreens can also indicate when it’s time to reapply after extensive sun exposure. Overall, the light-sensitive nature allows these materials to dynamically respond to UV light for functional and aesthetic purposes.
Photodegradation
Photodegradation is the process by which light breaks down materials. It occurs when light interacts with chemical bonds in a material, causing them to break. This process leads to degradation of the material’s chemical structure and properties.
There are several ways light can initiate photodegradation:
- Direct absorption of light energy by the chemical bonds in a material. This extra energy causes bonds to stretch and weaken.
- Excitation of electrons in a material into higher energy states. When they return to lower energy states, bonds can break.
- Production of reactive oxygen species like singlet oxygen that chemically attack and degrade materials.
- Generation of free radicals that initiate oxidation reactions and decomposition.
Factors that affect the rate of photodegradation include light intensity, wavelength, temperature, oxygen concentration, and the inherent chemical structure of the material. Materials like plastics, dyes, and pharmaceutical drugs are particularly susceptible.
Photodegradation can be useful for breaking down pollutants but detrimental when it degrades beneficial materials like plastics prematurely. Understanding the photodegradation mechanisms allows strategies like UV stabilizers and antioxidants to mitigate harmful effects.
Bleaching
Sunlight can have a bleaching effect on many materials, especially natural fabrics like cotton, linen, and wool. The sun’s ultraviolet (UV) rays break down the pigment molecules in fabric dyes, causing them to fade over time when exposed to sunlight. This is why continual sun exposure causes colored fabrics to gradually become lighter and more washed out in appearance.
The bleaching effect of sunlight is accelerated when fabric is soaked in water. The moisture helps the UV rays penetrate deeper into the fabric to degrade more pigment. Wet fabrics dried in direct sunlight often show obvious bleaching effects. Chlorine bleach produces a chemical bleaching effect in a similar process of destroying fabric pigments.
Wood exposed to the sun’s UV rays also suffers bleaching effects over time. Lignin, the compound responsible for rich natural wood colors, deteriorates and leaches out from continual UV exposure. This causes the wood to turn grey and appear weathered. Just like with fabric, wood bleaching is heightened when the material is wet, as the moisture carries the UV rays deeper into the wood.
To reduce sunlight bleaching, fabrics and wood can be treated with UV-absorbing chemicals. Keeping materials shaded or covered when not in use will also minimize bleaching. However, some bleaching effect is inevitable over decades of sun exposure.
Photosynthesis
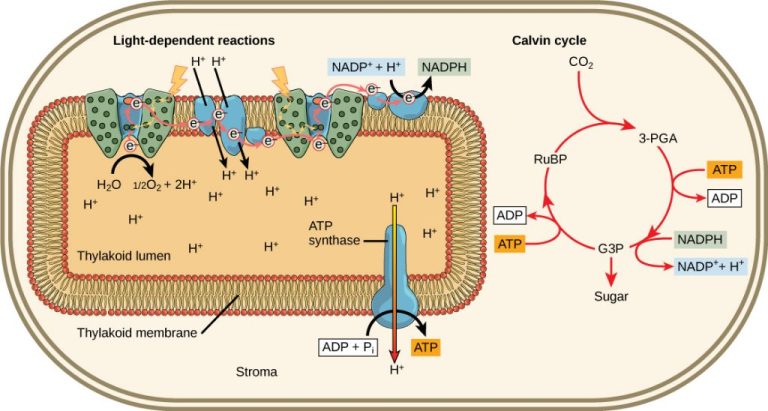
Light plays a crucial role in photosynthesis, the process by which plants convert light energy into chemical energy. Photosynthesis occurs in chloroplasts, specialized structures containing chlorophyll pigments that absorb light energy.
When sunlight hits the leaves, the chlorophyll absorbs the red and blue wavelengths while reflecting green wavelengths, giving leaves their green color. The absorbed light provides energy to convert carbon dioxide and water into carbohydrates like glucose. Oxygen is released as a byproduct.
The light reactions of photosynthesis involve photolysis, where water is split to liberate oxygen, and the generation of ATP and NADPH. These energy carriers are then used in the Calvin cycle reactions to fix carbon dioxide into sugar molecules.
Overall, photosynthesis converts light energy into chemical bonds that can be used as an energy source by plants. The creation of carbohydrates and oxygen through photosynthesis provides energy and oxygen not just for plants but for all aerobic life on Earth. Thus, sunlight enables a vital process that sustains the biosphere.
Vitamin D Synthesis
Sunlight plays a crucial role in the synthesis of vitamin D in the human body. Vitamin D is essential for regulating calcium absorption and promoting bone growth and maintenance. When skin is exposed to sunlight, specifically ultraviolet B (UVB) radiation, this triggers vitamin D synthesis.
Here’s how it works: UVB radiation penetrates the skin and converts cholesterol in the epidermis layer into previtamin D. Previtamin D is then converted into vitamin D3, otherwise known as cholecalciferol. Vitamin D3 travels through the bloodstream to the liver, where it undergoes hydroxylation to become 25-hydroxyvitamin D. Finally, the kidneys convert this form into the active form of vitamin D called calcitriol.
Calcitriol helps regulate calcium and phosphorus absorption in the intestines. It also enables bone mineralization by promoting the absorption of calcium into bone. Without sufficient vitamin D and calcium absorption, bone growth and strength may suffer over time.
Exposure to UVB radiation is the most efficient way to meet vitamin D needs. Dietary sources alone are often inadequate, especially during winter months or for people living in northern latitudes with less sun exposure. Given the importance of sunlight for vitamin D synthesis, many doctors recommend 10-15 minutes of unprotected sun exposure two to three times per week.
Suntans
When skin is exposed to UV radiation from sunlight, it causes the skin to produce more melanin, which is the pigment that gives skin its tan color. Melanin helps protect deeper layers of skin from UV damage. Here’s how the process works:
Melanocytes are cells in the skin’s epidermis layer that produce melanin. When melanocytes are exposed to UV rays, they ramp up melanin production as a defense mechanism. The melanin pigment absorbs and scatters UV radiation, blocking it from penetrating deeper and causing damage.
The melanin is transferred from melanocytes to keratinocytes, the most common type of cell found in the epidermis. As the keratinocytes populate the skin’s surface, the additional melanin they contain gives skin a darker, tan appearance.
This tan fades over time as the keratinocytes naturally slough off and are replaced by new cells. The tan provides moderate UV protection but is not a substitute for sunscreen, since it has a limited ability to block UV radiation.
Sunburn
One way light changes materials is by causing sunburns on human skin. Sunburns are caused by overexposure to ultraviolet (UV) rays from the sun. There are three types of UV rays: UVA, UVB, and UVC. UVA rays penetrate deep into the skin and cause aging and wrinkling. UVB rays burn the outer layers of skin and are the primary cause of sunburns. UVC rays are filtered out by the earth’s ozone layer before reaching us.
When UVB rays hit skin, they excite DNA molecules in skin cells, causing mutations. The cell recognizes this damage and initiates apoptosis, or programmed cell death. This causes the top layers of skin to peel off in a sunburn. UVB also stimulates melanin production, which creates a tan. Melanin helps block UV radiation from penetrating deeper and causing more DNA damage.
While sun exposure is healthy in moderation, too much UV radiation leads to sunburns. Sunburns damage DNA, accelerate skin aging, and increase skin cancer risk. Using sunscreen, covering up, avoiding peak hours, and gradually building tolerance can help prevent harmful UV exposure.
Cataracts
Cataracts are a clouding of the natural crystalline lens in the eye that affects vision. Over time, exposure to certain wavelengths of sunlight can cause proteins in the lens to clump together and discolor, forming a cataract. This blocks light from properly passing through the lens to the retina, causing blurred vision.
Ultraviolet (UV) radiation seems particularly instrumental in cataract formation. UV light has shorter wavelengths and higher energy that can damage lens proteins. As we age, the lens also loses the ability to filter out UV rays. This cumulative exposure to UV radiation over many years allows photochemical reactions to take place in the eye, especially in the lens, leading to protein clumping and cataract formation. The risk and severity of cataracts increases with more sun exposure.
Cataracts usually develop slowly over many years. They often start with cloudy spots in parts of the lens that spread until the entire lens becomes opaque. The clouded vision makes it difficult to read, drive, or see colors properly. While cataracts cannot spread from eye to eye, most occur in both eyes. Surgery to remove and replace the cloudy natural lens with an artificial lens is the only treatment, but it is highly effective in restoring vision. Wearing sunglasses that block 100% of UV rays helps prevent and delay cataracts.
Conclusion
Light can transform materials in many different ways, some beneficial and some harmful. As we’ve seen, certain materials like photochromic lenses darken when exposed to light, providing helpful eye protection. On the other hand, light can degrade or bleach materials through photodegradation, breaking down dyes, plastics, and other materials over time. Photosynthesis uses light energy to transform carbon dioxide and water into sugars and oxygen, providing energy for plants and producing the oxygen we breathe. Light also triggers the synthesis of vitamin D in our skin for bone health. At the same time, excessive light exposure can lead to suntans, sunburns, and cataracts in our eyes. Ultimately, light is an incredibly powerful force that can profoundly change the atomic structure and properties of matter, for better or worse. Understanding these photochemical processes allows us to utilize light’s benefits while protecting ourselves from its potential damages.