What Spectrum Is Radiation?
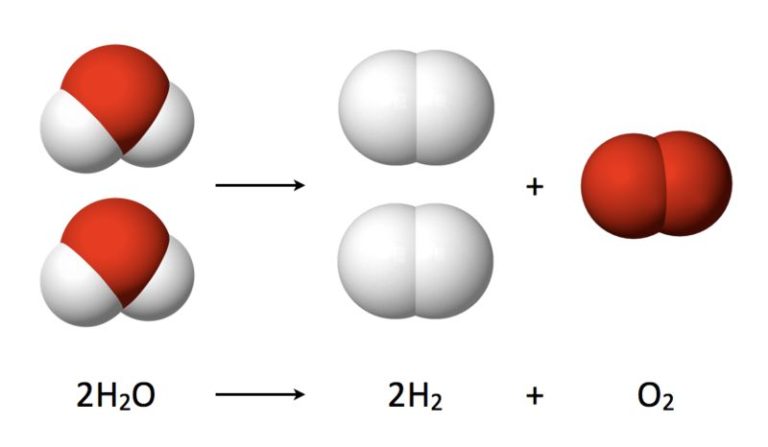
Radiation refers to energy that travels in the form of waves or particles. There are different types of radiation that exist on the electromagnetic spectrum. The electromagnetic spectrum encompasses all electromagnetic radiation and arranges them according to frequency and wavelength. The main types of radiation include radio waves, microwaves, infrared, visible light, ultraviolet, x-rays, and gamma rays.
Radiation has many practical applications in our everyday lives. For example, radio waves are used for radio and television broadcasting, microwaves are used for cooking food, infrared radiation is used in night vision devices, and x-rays and gamma rays are used in medical imaging. Radiation can also come from natural sources like the Sun and radioactive elements. Overall, radiation serves many important purposes across science, technology, and medicine.
Electromagnetic Spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. Electromagnetic radiation is energy that travels and spreads out as it goes – the visible light that comes from a lamp and the radio waves that come from a radio station are two types of electromagnetic radiation. The electromagnetic spectrum encompasses all possible wavelengths or frequencies of electromagnetic energy.
Electromagnetic waves are categorized according to their frequency and wavelength. The electromagnetic spectrum is generally divided into seven regions, in order of decreasing wavelength and increasing energy and frequency. The common designations are: radio waves, microwaves, infrared (IR), visible light, ultraviolet (UV), X-rays and gamma rays.
Radio waves have frequencies up to around 300 gigahertz. Microwaves have frequencies up to around 300 megahertz. Infrared radiation lies between the visible and microwave portions of the electromagnetic spectrum, with wavelengths from about 700 nanometers to 1 millimeter. Visible light is the portion of the electromagnetic spectrum that is visible to the human eye, with wavelengths from about 380 nanometers to about 740 nanometers. Ultraviolet (UV) light has shorter wavelengths than visible light. X-rays have shorter wavelengths than UV rays. Gamma rays have the shortest wavelengths and the highest frequency and energy in the electromagnetic spectrum.
Radio Waves
Radio waves are a type of electromagnetic radiation with wavelengths in the electromagnetic spectrum longer than infrared light. Radio waves have frequencies ranging from as low as 30 Hertz to as high as 300 Gigahertz, corresponding to wavelengths of 10 kilometers to 1 millimeter. They have unique properties that make them useful for communications and other applications.
Some key properties of radio waves include:
- Radio waves can travel long distances and penetrate most objects, though not as well as microwaves.
- Radio waves exhibit diffraction – they bend around obstacles and follow the curvature of the Earth.
- Radio waves can carry information by modulating properties like amplitude, frequency, and phase.
- Radio waves at different frequencies exhibit different propagation characteristics.
Radio waves have many important uses today, including:
- Broadcast radio – AM and FM radio stations use radio waves to transmit audio signals and programming.
- Television – Analog and digital TV signals are transmitted over the air via radio waves.
- Wireless networks – WiFi, Bluetooth, and cellular networks rely on radio waves to send data wirelessly.
- Radio astronomy – Radio telescopes detect radio waves from space to study astronomical phenomena.
- Radar – Radio waves are used in radar systems to detect objects and determine their position and velocity.
- Satellite communications – Satellites transmit data for communications, GPS, and weather monitoring using radio waves.
Microwaves
Microwaves are a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter. They have frequencies between 300 MHz (300 million cycles per second) and 300 GHz (300 billion cycles per second).

Some key properties of microwaves include:
- Microwaves can penetrate through some materials like plastic, paper, and some ceramics.
- They are reflected by metals.
- Microwaves cause dielectric heating in water and fat, which is how microwave ovens are able to cook food.
- Microwaves are absorbed by the atmosphere and do not travel long distances like radio waves.
Microwaves have many practical uses:
- Microwave ovens use microwave radiation to heat and cook food.
- Microwave relays are used for transmitting information, like telephone calls, television programs, and computer data.
- Microwaves are used in radar technology to detect objects and determine their distance.
- Microwave heating is used in industrial processes.
- Microwave spectroscopy is used to study the structure of molecules by their interaction with microwaves.
Infrared Radiation
Infrared radiation is part of the electromagnetic spectrum that sits between visible light and microwaves. It has longer wavelengths than visible light, but shorter wavelengths than microwaves.
Infrared radiation has some unique properties that make it useful for many applications:
- Infrared is invisible to the human eye, but can be detected as heat. Objects emit infrared radiation proportional to their temperature.
- Infrared radiation is able to penetrate haze, fog, clouds, and smoke better than visible light. This makes infrared imaging useful for astronomy and Earth observation from space.
- Most molecules absorb specific frequencies of infrared light which correspond to their natural molecular vibrations. This allows infrared spectroscopy to be used to study and identify chemical substances.
- Night vision technologies make use of infrared’s ability to penetrate low-light conditions. They detect infrared emitted by objects and living things, allowing them to be seen in very dim light.
Some common uses and applications of infrared radiation include:
- Infrared thermal imaging: Detecting infrared radiation emitted by objects allows their surface temperature to be measured remotely. This is used widely in military, security, medicine, and industry.
- Infrared spectroscopy: Identifying materials by their unique molecular fingerprint in the infrared spectrum. Used for chemistry, atmospheric science, art analysis, and more.
- Infrared heating and cooking: Infrared is absorbed by food and objects, heating them directly. It is used in infrared saunas, broilers, and cooking.
- Infrared astronomy: Infrared telescopes can see distant stars and galaxies not visible in visible light.
- Infrared photography: Infrared light passes through some materials that block visible light, allowing unique infrared photography.
Visible Light
Visible light is the portion of the electromagnetic spectrum that is visible to the human eye. It has wavelengths between 380-700 nanometers. Visible light is perceived by the eye and interpreted in the brain as different colors based on its wavelength. The color perceived depends on the wavelength of the light. Violet light has shortest wavelengths around 380-450 nm while red light has the longest wavelengths, around 620-700 nm. Beyond these limits, the wavelengths are invisible to humans but can be detected by instruments and cameras.
Visible light plays an essential role in nature. Plants use visible light for photosynthesis. Chlorophyll absorbs mainly blue and red light and reflects green light, giving plants their green appearance. Many animals are also adapted to see visible light to find food, detect danger, communicate, and reproduce. The scattering of sunlight by molecules in the atmosphere gives the sky its blue color. Atmospheric conditions like dust, haze, and humidity can affect how visible light is attenuated and scattered as it travels through the air. At sunrise and sunset, the sun’s light has farther to travel through the atmosphere so more blue light is scattered away, causing the remaining light to appear orange and red.
Ultraviolet Radiation
Ultraviolet (UV) radiation is a higher energy, shorter wavelength radiation that is just beyond the visible spectrum. UV radiation ranges from 10nm to 400nm wavelength. There are three types of UV radiation: UVA, UVB, and UVC.
UVA makes up the majority of UV radiation that reaches the Earth’s surface. It can penetrate deep into the skin and is associated with skin aging and wrinkling. UVB is more energetic but does not penetrate as deep. It is primarily responsible for sunburns and skin cancer. UVC has the highest energy but is blocked by the ozone layer before reaching the ground. Exposure to UVC can cause severe burns and eye damage.
Overexposure to UV radiation has various health effects. It can lead to sunburn, skin cancer, premature skin aging, eye damage, and suppression of the immune system. UV exposure is the primary cause of melanoma and nonmelanoma skin cancers. It also contributes to eye diseases such as cataracts. Protection from UV can be achieved through shade, clothing, hats, sunglasses, and sunscreen.
X-Rays
X-rays are a form of ionizing radiation with wavelengths shorter than ultraviolet light but longer than gamma rays. X-rays have very high frequency and energy, allowing them to penetrate most materials including human tissue.
X-rays have some important properties that make them useful for medical imaging and other applications:
- They can pass through soft tissue and bone, allowing x-ray images to reveal internal structures.
- They are absorbed (or attenuated) at different levels by different materials, allowing x-rays to create contrast between things like bone, soft tissue, and air.
- Their ionizing ability can damage or kill cells by breaking molecular bonds.
Some of the most common medical uses of x-rays include:
- X-ray imaging – Using low dose x-rays to create images of bones and organs inside the body. This allows doctors to view and assess broken bones, tumors, pneumonia, etc.
- CT scanning – Combining multiple x-ray images taken around a rotating object to generate cross-sectional views inside the body.
- Fluoroscopy – Using continuous or pulsed x-rays to obtain real-time moving images of structures inside the body.
- Angiography – Using contrast x-ray imaging to view blood vessels and organs.
While invaluable for medical diagnosis, x-rays do carry some risks due to their ionizing radiation. Doctors take precautions to limit patient exposure to the lowest radiation doses that provide the needed diagnostic information.
Gamma Rays
Gamma rays have the highest frequency and shortest wavelength in the electromagnetic spectrum. They typically have frequencies above 10 exahertz (1019 Hz), corresponding to wavelengths of less than 10 picometers. Gamma rays consist of high energy photons with energies from tens of thousands to millions of electronvolts.
Gamma rays are produced by extremely energetic events and processes in the universe, such as supernovas, neutron stars, and black holes. On Earth, they can be produced by nuclear reactions, nuclear decay, and high energy particle collisions.
Some properties of gamma rays include:
- Extremely high energy and frequency
- High penetrating power, able to pass through most materials
- Ionizing radiation, able to strip electrons from atoms and molecules
- Travel at the speed of light
Gamma rays have several important uses:
- Medical imaging and radiation therapy
- Security screening of luggage and cargo
- Sterilization of medical equipment
- Studying high energy astrophysical processes
- Nuclear weapons testing
Gamma rays require heavy shielding and careful handling due to their high energy and penetrative power. But their unique properties also make them invaluable for many applications in medicine, industry, security, and research.
Ionizing vs Non-Ionizing Radiation
Radiation exists on a spectrum, ranging from radio waves on one end to gamma rays on the other. The key difference between ionizing and non-ionizing radiation lies in their ability to directly damage DNA. Ionizing radiation has enough energy to remove electrons from atoms or molecules, creating charged particles called ions. Non-ionizing radiation does not have enough energy to ionize atoms.
Ionizing radiation includes ultraviolet rays, x-rays, and gamma rays. These rays have very high frequencies and short wavelengths, allowing them to penetrate human tissue and damage DNA. Exposure to ionizing radiation can lead to cancer, burns, radiation sickness, and genetic mutations if the dose is high enough. However, ionizing radiation also has medical uses, like x-rays and radiation therapy for cancer treatment.
Non-ionizing radiation includes radio waves, microwaves, infrared light, and visible light. These types of radiation have longer wavelengths and lower frequencies, so they do not cause direct DNA damage. However, exposure to large amounts of non-ionizing radiation can still potentially cause tissue heating and other biological effects. Overall, non-ionizing radiation is generally less harmful than ionizing radiation.
In summary, ionizing radiation has enough energy to ionize atoms and damage cell DNA, causing more severe biological effects. Non-ionizing radiation does not directly ionize atoms or damage DNA, though exposure to high levels can still potentially cause some heating effects in tissue.