What Light Do Solar Panels Absorb?
Solar panels are devices that convert sunlight into electricity. They work through the photovoltaic effect, which causes materials exposed to light to release electrons. When these free electrons are captured, an electric current results that can be used as electricity.
Solar panels are typically made of silicon arranged in a grid-like pattern on the panel’s surface. When sunlight shines on the solar panel, photons from the sunlight are absorbed by the silicon in the cells. This energizes the electrons and causes them to be released. The solar cells are wired together in a circuit, creating an electric field that forces the electrons to flow in one direction, creating a Direct Current or DC which is collected and supplied for use. This flow of electrons is basically electricity that can then be used to power electrical loads.1
By connecting multiple solar panels together, more electricity can be generated to meet electrical demands. The panels are modular, so it’s easy to add more panels to increase capacity as needed. This makes solar power a versatile and scalable source of renewable energy.
Solar Spectrum
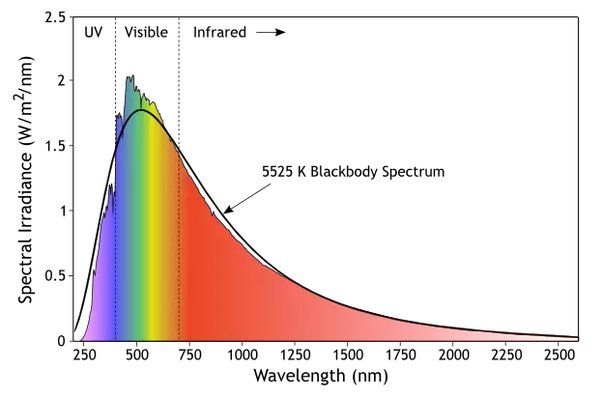
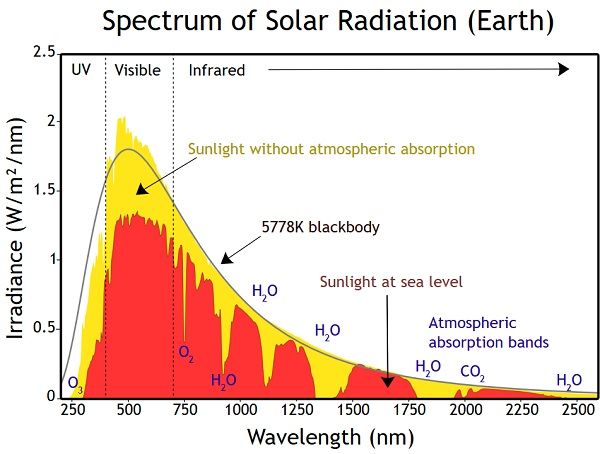
The solar spectrum refers to the electromagnetic radiation emitted by the sun across a wide range of wavelengths. This includes visible light that humans can see, as well as ultraviolet light, infrared light, and other forms of electromagnetic radiation. According to Green Rhino Energy (http://www.greenrhinoenergy.com/solar/radiation/characteristics.php), the energy in solar irradiation comes in wavelengths ranging from 0.2 microns to over 2.5 microns.
The spectrum is composed of different wavelengths, each with their own amount of energy. Shorter wavelengths like ultraviolet have higher frequencies and more energy, while longer wavelengths like infrared have lower frequencies and less energy. Visible sunlight that humans perceive as colors ranges from violet (around 380 nanometers) to red (around 740 nanometers).
When sunlight passes through Earth’s atmosphere, some wavelengths are absorbed or scattered more than others. According to the University of Rochester (https://www.pas.rochester.edu/~blackman/ast104/spectrumsun.html), this filtering effect is why the solar spectrum has dark absorption lines, known as Fraunhofer lines, overlaid on the continuum of wavelengths.
Photovoltaic Effect
The photovoltaic effect refers to the process by which solar cells convert sunlight into electricity. When photons from sunlight strike the solar cell, they transfer their energy to the electrons in the semiconductor material of the solar cell. This energy causes the electrons to break free from their atoms and flow through the material to produce electric current.
Specifically, in a typical silicon solar cell, photons from sunlight excite electrons in the silicon from the valence band to the conduction band, creating mobile electron-hole pairs. The built-in electric field of the p-n junction separates the electron-hole pairs before they can recombine. The electrons are attracted to the n-type side and the holes to the p-type side, creating a voltage difference that drives electric current when the solar cell is connected to a load.
The photovoltaic effect was first observed by French physicist Alexandre-Edmond Becquerel in 1839 when he found that illuminated platinum electrodes submerged in electrolyte would produce small amounts of electric current. Building on Becquerel’s discovery, researchers over the next century continued to study the photovoltaic effect in various materials, leading to the invention of the first practical solar cell by Bell Labs researchers Daryl Chapin, Calvin Fuller, and Gerald Pearson in 1954. Today, the photovoltaic effect is key to generating electricity from sunlight in solar panels and solar-powered devices.[1]
[1] https://www.sciencedirect.com/topics/engineering/photovoltaic-effect
Solar Cell Materials
The most common materials used for solar cells are silicon, thin films, and organic photovoltaics. Silicon comes in three main types: monocrystalline, polycrystalline, and amorphous. Monocrystalline silicon is the most efficient but also most expensive. It’s made out of silicon ingots sliced into perfectly structured wafers. Polycrystalline silicon has lower efficiency than monocrystalline but is less expensive. It’s made from melted and re-solidified silicon fragments. Amorphous silicon has the lowest efficiency but can be deposited on substrates to make thin flexible cells. Thin film materials like cadmium telluride (CdTe) and copper indium gallium selenide (CIGS) require less semiconductor material and are cheaper but less efficient than silicon cells. Organic photovoltaics like plastic solar cells are inexpensive and flexible but have very low efficiency levels currently.
Sources:
- https://www.energy.gov/eere/solar/solar-photovoltaic-cell-basics
- https://g2voptics.com/photovoltaics-solar-cells/solar-cell-materials/
Absorption of Light
Solar panels are made up of photovoltaic cells, which contain semiconducting materials like silicon that enable the photovoltaic effect. When sunlight hits these solar cells, the photons are absorbed by the semiconducting material, causing electrons to break free from their atoms. This generates positively charged electrons and negatively charged holes that result in an electric field across the solar cell. The electrons move to one side of the cell, while the holes move to the other side, allowing current to flow when the solar cell is connected to an external load (Solar Photovoltaic Cell Basics).
The amount of light a solar cell can absorb depends on the band gap energy of the semiconductor. The band gap determines the threshold wavelength of light that can be absorbed. Photons with energy greater than the band gap are absorbed, while those below the band gap pass through the material without being absorbed (Solar cells that make use of wasted light). Most solar panels are made of crystalline silicon, which has a band gap of 1.1 eV, allowing it to absorb photons in the visible and UV light spectrum.
Wavelengths Absorbed by Solar Panels
Solar panels are designed to absorb sunlight efficiently across the visible light spectrum and some of the ultraviolet spectrum. Visible light wavelengths range from about 380-750 nm. The most common solar cell material, crystalline silicon, has a peak light absorption range of 300-1100 nm with maximum efficiency between about 500-600 nm in the visible light spectrum.1 This allows solar panels to absorb violet, blue, green, yellow, orange and red visible light from the sun most efficiently. Solar panels can also absorb some ultraviolet light, primarily in the range of 100-400 nm wavelengths. However, infrared light above ~750 nm is not utilized as well in typical solar cells.
The reason silicon solar cells are designed for peak visible light absorption is because the sun’s radiation peaks in the visible range, centered around green light at about 550 nm. Solar panel materials and junction depths are engineered to optimize light capture across the highly energetic visible photons. Ultraviolet photons have even higher energy, but solar UV intensity is lower, so less overall energy is available to collect. On the infrared end, photons have too little energy to free electrons in the solar cell material.
By tuning solar panels for efficient absorption across the visible spectrum and some UV, where the sun’s intensity peaks, the most solar energy can be harvested. The photovoltaic effect, which converts absorbed photons into electrical current, works best with visible and UV rays at the heart of the solar spectrum.
Infrared and Light Absorption
Solar panels are generally less efficient at converting longer wavelengths of light, like infrared, into electricity. Most solar panels are made of silicon, which has an optimal absorption range in the visible and ultraviolet light spectrums
As the wavelength of light increases into the infrared range, silicon becomes less efficient at absorbing photons and converting them into electrical current. This is because the energy of infrared photons is below the bandgap energy of silicon, making them more likely to pass through the material without being absorbed.
While infrared light is plentiful from the sun, most commercial silicon solar cells are not optimized to utilize it. Some novel solar cell designs aim to better absorb infrared wavelengths by using lower-bandgap materials like gallium arsenide. But silicon remains the dominant material for its balance of efficiency and cost-effectiveness.
Overall, the conversion efficiency of solar panels decreases for light at the red end of the visible spectrum and beyond into infrared. But panels can still produce electricity from infrared light, just at lower efficiencies than shorter wavelength visible light that better matches silicon’s absorption profile.
Source: https://www.thinkepic.com/solar/can-solar-panels-use-ultraviolet-or-infrared-light/
Panel Orientation
For maximum sunlight absorption, solar panels should face true south in the northern hemisphere. Facing south allows the panels to receive direct sunlight throughout the day as the sun tracks across the sky. According to Sunrun, arrays facing south can improve energy output by up to 30% compared to non-optimal orientations.
Gilbert Michaud, assistant professor at Ohio State University, stated “In the northern hemisphere, it’s optimal for your solar panels to be facing south.” This south-facing orientation maximizes the number of daylight hours the panels receive direct sunlight.
According to Arka360, “In the northern hemisphere, facing south is the most effective orientation for solar panels.” The optimal solar panel direction depends on latitude, but south is generally best for absorbing the most sunlight.
Effects of Weather
Solar panels are designed to absorb sunlight in order to generate electricity. However, weather conditions can impact the amount of sunlight that reaches the panels and thus affect their efficiency. Two major weather factors that influence solar panel efficiency are cloud cover and air pollution.
Clouds block direct sunlight from reaching the solar panels. Even on cloudy days, panels can still produce some electricity from diffuse sunlight passing through cloud cover. But the power output is diminished compared to sunny days. Thick storm clouds can reduce solar production to just 10-25% of the rated panel capacity. Partial cloudiness also decreases output from momentary shade passing over the panels 1.
Air pollution and smog also cut down the intensity of sunlight reaching the solar array. Particulate matter in polluted air scatters and absorbs incoming solar radiation. Studies show that higher pollution days directly correspond with lower solar panel yields. Dirty air can decrease electricity production by solar panels by up to 25% compared to clean air conditions 2.
Conclusion
In conclusion, the wavelengths of visible light that solar panels are designed to absorb depend on the semiconductor materials used in the solar cells. Silicon cells can absorb wavelengths up to 1100nm in the near-infrared, while perovskite and GaAs cells can absorb light further into the infrared spectrum. Proper panel orientation is crucial to maximize light absorption. Research shows that light absorption can be enhanced by using nanoparticles, anti-reflection coatings, and light-trapping techniques. In the future, novel semiconductor materials and optical engineering will enable solar panels to absorb an even broader spectrum of light, improving efficiency and electricity generation.