What Is The Process Of Converting Energy Called?
What is Energy Conversion?
Energy conversion is the process of changing energy from one form to another. For example, a wind turbine converts the kinetic energy of wind into electrical energy. An internal combustion engine converts the chemical energy stored in fuel into mechanical energy or motion.
Some common energy conversion processes include:
- Chemical to thermal – Burning fuel such as natural gas, gasoline, or wood releases heat (thermal energy).
- Thermal to mechanical – Heat engines like internal combustion engines and steam turbines use thermal energy to generate mechanical power.
- Mechanical to electrical – Electric generators convert mechanical energy into electricity using electromagnetic induction.
- Radiant to thermal – Solar cells convert sunlight into electricity, with heat as a byproduct.
- Gravitational potential to kinetic – Hydropower uses the movement of water from high to low elevations to spin turbines.
Energy is never created or destroyed, but can change forms. Energy conversion enables useful work and powers modern civilization.
Forms of Energy
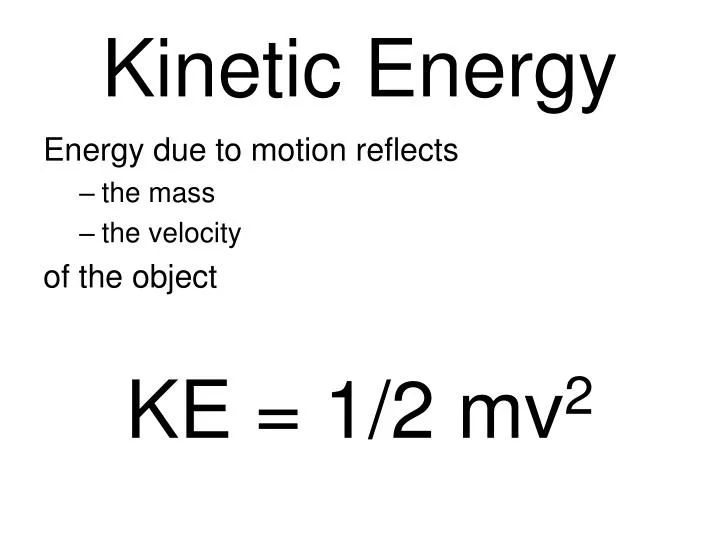
Energy exists in various forms that can be categorized into two main types: potential energy and kinetic energy. Potential energy is stored energy possessed by an object due to its position or chemical composition. Kinetic energy is the energy of motion that a moving object possesses. There are several common forms of potential and kinetic energy:
Potential Energy
– Gravitational Potential Energy: The energy an object possesses due to its height above the ground. The higher the object, the greater its gravitational potential energy.
– Elastic Potential Energy: The energy stored in elastic materials or objects by the application of a force. Compressed springs and stretched rubber bands have elastic potential energy.
– Chemical Potential Energy: The energy stored in the bonds between atoms and molecules. This energy can be released during chemical reactions.
Kinetic Energy
– Radiant Energy: The energy carried by electromagnetic radiation such as light, radio waves, x-rays, and gamma rays.
– Thermal Energy: The internal energy possessed by an object due to the random motion of its atoms and molecules. Also called heat energy.
– Mechanical Energy: The sum of an object’s kinetic and potential energy. The energy associated with the motion and position of an object.
– Electrical Energy: The energy derived from electric charges or fields. Used to power electrical devices.
– Sound Energy: The energy associated with the vibration or movement of atoms that is carried through gases, liquids and solids in waves.
Laws of Thermodynamics
The laws of thermodynamics describe the relationships between thermal energy, heat, and other forms of energy. There are two main laws of thermodynamics:
The first law states that energy can neither be created nor destroyed in an isolated system. This is known as the law of conservation of energy. This means that energy can only change forms – for example, heat energy can be converted into kinetic energy. The total amount of energy in a closed system remains constant.
The second law of thermodynamics states that the entropy of an isolated system always increases over time. Entropy is a measure of disorder and randomness. As an isolated system evolves, it tends to move towards a state of higher entropy. This law implies that most energy transformations are not completely efficient, as some energy is dissipated as waste heat. This lost energy can never be fully recovered or converted into useful work.
These fundamental thermodynamic laws place limits on how efficiently energy can be harnessed and converted. Understanding these laws is key to improving energy conversion devices and systems.
Common Energy Conversions
There are several common types of energy conversions that happen regularly in everyday life and technology applications:
Chemical to Thermal
Chemical energy stored in the molecular bonds of substances like fossil fuels, food, and batteries can be converted into thermal energy through exothermic chemical reactions. For example, combustion of gasoline in a car engine converts the chemical energy to heat.
Chemical to Mechanical
Chemical energy can also be converted into kinetic energy and mechanical work. For instance, metabolizing food provides energy that allows human muscles to move. Fuel combustion in engines also converts chemical energy into mechanical motion.
Electrical to Thermal
Electric current flowing through a resistor converts electrical energy into heat dissipation according to Joule’s first law. Common appliances like incandescent lights, stoves, heaters all rely on this electricity to heat conversion.
Electrical to Mechanical
Electrical energy can be converted into rotational kinetic energy in electric motors. This allows electricity to power fans, pumps, electric vehicles and more. Generators can also convert mechanical energy into electricity.
Energy Conversion Devices
There are several common types of devices that are designed to convert one form of energy into another:
Steam Turbine
A steam turbine converts the heat energy in steam into mechanical energy by spinning a turbine to generate electricity. In a steam power plant, heat from burning fossil fuels boils water to produce high-pressure steam that flows through the turbine blades, causing rotation. This spinning turbine is connected to a generator which converts the mechanical rotation into electrical energy through electromagnetic induction.
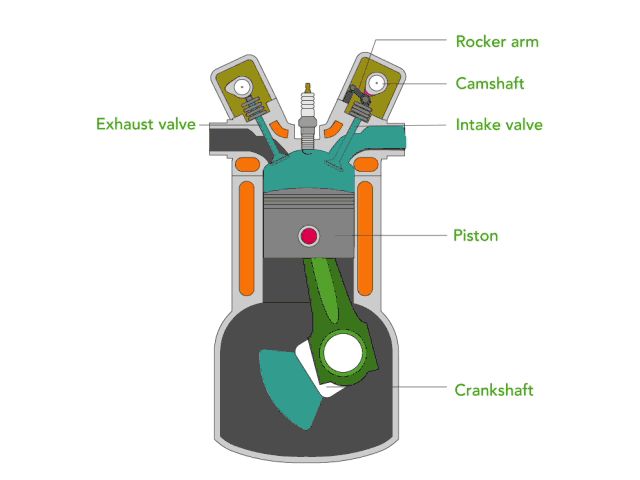
Internal Combustion Engine
An internal combustion engine, like in a car, converts chemical potential energy in fuel into thermal energy by burning the fuel inside a combustion chamber. This rapid expansion of gases pushes a piston to turn a crankshaft, converting the thermal energy into mechanical energy that can be used to power the wheels.
Battery
A battery is a device that converts chemical energy into electrical energy. Chemical reactions inside the battery produce a flow of electrons from the anode to the cathode through the external circuit, generating an electric current. Batteries allow chemical energy to be stored and released on demand in a portable, controlled way.
Fuel Cell
A fuel cell converts the chemical energy in a fuel like hydrogen into electricity through an electrochemical reaction. Hydrogen fuel fed into the anode reacts with oxygen at the cathode to produce water, generating an electric current in the process. Fuel cells provide very efficient and clean energy conversion.
Solar Cell
Solar cells, also called photovoltaic cells, convert sunlight directly into electricity. Photons from the sun knock electrons loose in the semiconductor material of the solar cell, causing electron flow and an electric current. Solar cells are used in solar panels to harness the sun’s energy for electricity.
Efficiency of Conversion
The efficiency of an energy conversion process refers to what percentage of the initial energy is converted into usable energy. For example, when converting chemical energy in gasoline to mechanical energy in a car engine, not all of the energy content of the gasoline is turned into usable work. Some energy is always lost as heat, sound, friction, etc. These energy losses due to the limitations of conversion processes mean that the efficiency is always less than 100%.
Different energy conversion devices have varying efficiencies based on the processes involved. For example, a typical gasoline car engine may have an efficiency around 25%, while a diesel engine can reach 40% efficiency. Power plants that convert the chemical energy in coal to electrical energy generally operate in the 30-50% efficiency range. The theoretical maximum efficiency possible for a standard thermal power plant is around 60% according to the laws of thermodynamics.
There are always energy losses and waste when converting energy from one form to another. The second law of thermodynamics states that no energy conversion process can be 100% efficient. Common energy losses include:
- Waste heat and exhaust – Heat is a byproduct of most energy conversions.
- Friction – Mechanical systems always have some friction converting useful work to heat.
- Electrical resistance – Electrical currents face resistance in wires producing waste heat.
- Sound and vibration – Noise is a form of wasted energy.
- Incomplete chemical reactions – Not all fuel may undergo complete combustion.
Since no process is perfectly efficient, improving conversion efficiencies is an important area of technology development. Even small gains in efficiency can lead to significant energy savings. Using more efficient devices and minimizing energy waste allows us to get more usable energy output from the world’s limited energy resources.
Importance of Efficient Conversion
Increasing the efficiency of energy conversion processes is critical for several reasons. Most importantly, it reduces energy waste. When energy is converted from one form to another, there is inevitably some loss of usable energy in the process, usually in the form of heat. The lower the efficiency, the more energy is wasted. By improving conversion efficiency, less primary energy needs to be generated to achieve the same useful output.
More efficient conversion also provides important environmental benefits. Since less energy is wasted, fewer fossil fuels may need to be burned to produce a given amount of usable energy. This reduces air pollution, greenhouse gas emissions and other impacts associated with fossil fuel extraction and use. Greater efficiency thereby lessens the environmental footprint of meeting society’s energy needs.
Efficient conversion is also key to getting the most out of renewable energy sources like solar and wind power. Since these sources can’t be controlled as easily as fossil fuels, it’s important that their maximum potential energy be extracted and converted as efficiently as possible into usable forms of energy.
So whether it’s reducing the amount of coal burned at a power plant, or getting more usable electricity out of sunlight, improving the efficiency of energy conversion plays a major role in building a more sustainable energy system.
Improving Efficiency
As energy demand continues to rise globally, improving the efficiency of energy conversion processes becomes increasingly important. There are several ways we can enhance efficiency through technological advances, proper maintenance, and energy storage.
On the technology front, developing better materials and systems that waste less energy to heat, friction, and other losses can boost efficiency. For example, improving insulation, lubrication, precision manufacturing, and more advanced electronics can reduce wasted energy across many conversion devices. New technologies like cogeneration and fuel cells also promise more efficient conversion.
Proper maintenance is another key factor. As equipment ages, efficiency tends to decline due to component degradation, leaks, accumulation of deposits, and other issues. Regular upkeep, inspections, cleaning, part replacement, and overhauls can optimize performance and extend asset lifetime.
Finally, energy storage plays a major role. Converting energy when supply exceeds demand and storing it for later use improves overall efficiency. Batteries, pumped hydro, compressed air, hydrogen, and other storage methods allow recapturing energy rather than wasting it.
With a combination of technological innovation, vigilant maintenance, and energy storage solutions, the future looks bright for more efficient energy conversion.
Future of Energy Conversion
As the world’s energy needs continue to grow, there is increasing focus on developing new and improved technologies for energy conversion. Some key areas of focus for the future include:
Developing new technologies – Scientists and engineers are working to discover, research and develop new materials, devices and processes to make energy conversion more efficient, affordable and sustainable. This includes advances like organic electronics, nanotechnology, biomimetics, and more.
Renewable energy sources – There is major emphasis on improving technology to convert renewable sources like solar, wind, tidal, geothermal and biofuels into usable energy. Renewable sources can provide sustainable alternatives to fossil fuels for energy conversion.
Decentralized systems – Rather than relying solely on large centralized power plants, the future may see more distributed energy systems that allow small-scale conversion at the point of use. This can reduce transmission losses and increase resilience.
With focused research, innovation and adoption of new technologies, the future of energy conversion looks bright. More efficient, sustainable processes for converting energy will be key to meeting the world’s growing energy demands.
Conclusion
In summary, energy conversion is the process of changing energy from one form to another. The laws of thermodynamics govern energy conversion, stipulating that while energy cannot be created or destroyed, some is always lost as heat. Common energy conversions include chemical to thermal, chemical to electrical, thermal to mechanical, and more.
Energy conversion takes place through various devices like generators, fuel cells, turbines, and engines. The efficiency of conversion depends on factors like heat loss and friction, and it is improved through methods like insulation and reducing moving parts. Efficient conversion is crucial for reducing waste, costs, and environmental impact.
As we advance technologically, new techniques and materials allow us to improve conversion efficiency. With global energy needs rising, efficient conversion will become increasingly vital. Though no process is 100% efficient, striving for maximum efficiency will allow us to get the most out of our energy sources while conserving resources for the future.