What Is The Meaning Of Heat Transfer Words?
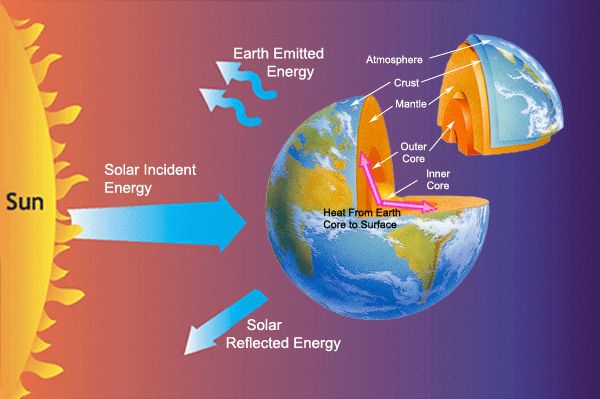
Heat transfer is the process of thermal energy moving from one system to another due to a temperature difference. It plays a vital role in many engineering systems and natural processes. Understanding heat transfer is crucial for designing and optimizing systems like engines, air conditioners, chemical processing plants, and even living organisms.
There are three main mechanisms of heat transfer: conduction, convection, and radiation. Conduction is the transfer of heat between objects in direct contact with each other. Convection utilizes the motion of fluids to transfer heat, while radiation works through electromagnetic waves emitted by all objects proportional to their temperatures.
Some key terminology related to heat transfer includes:
- Thermal conductivity – a property that quantifies a material’s ability to conduct heat
- Convective heat transfer coefficient – used to calculate convection heat transfer rates
- Emissivity – a measure of how well an object emits radiant energy compared to a perfect blackbody
- Thermal resistance – quantifies the resistance to heat flow within and across objects
This overview of heat transfer introduces the main mechanisms and terminology involved. The following sections will explore each of these heat transfer processes and applications in more detail.
Conduction
Conduction is the transfer of heat between substances that are in direct contact with each other. It occurs when heat energy is transferred from particles with higher kinetic energy to adjacent particles with lower energy through direct interactions at a molecular level.
Materials that allow heat to easily flow through them are called thermal conductors. Metals like copper, aluminum, and silver are excellent conductors of heat. This is because their atomic structure allows heat energy to quickly pass between atoms. Materials that resist conduction are called thermal insulators. Insulators like plastic, rubber, and styrofoam slow down heat transfer because their molecular structure reduces interactions between neighboring atoms.
Examples of heat conduction include a spoon heating up when placed in a hot cup of coffee. The metal spoon readily conducts heat from the hot liquid it is in direct contact with. Another example is a cast iron skillet heating up on a stove burner. The metal pan absorbs the heat from the direct flame through conduction.
Convection
Convection refers to heat transfer that occurs between a surface and a moving fluid or gas. It involves the transfer of heat by the bulk, macroscopic movement of matter. There are two main types of convection:
Natural convection occurs when fluid motion is caused by temperature-induced differences in density and the action of gravity. For example, when water is heated in a pot, the hotter, less dense water at the bottom rises to the top while the cooler, denser water sinks to the bottom, creating circulation. This circulation allows heat to be distributed throughout the water.
Forced convection occurs when an external source causes the fluid motion, such as a fan or pump. Forced air furnaces and car radiators rely on fans to blow air over heated surfaces and enhance heat transfer. This allows more efficient heating or cooling compared to natural convection alone.
Both natural and forced convection play important roles in heat transfer processes like cooking, weather patterns, heating and cooling systems, and more. Convection is often utilized in engineering designs to enhance thermal performance.
Radiation
Thermal radiation refers to the emission of electromagnetic waves from all matter that has a temperature above absolute zero. Objects and surfaces emit thermal radiation constantly. The hotter something is, the more thermal radiation it emits at shorter wavelengths. Thermal radiation does not require particles to transfer the heat and can propagate through vacuum (as opposed to conduction and convection).
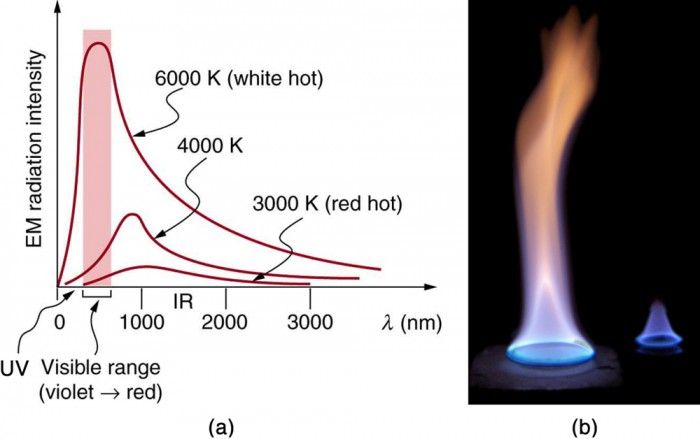
The radiative properties of materials refer to how well they emit, reflect, transmit or absorb thermal radiation. These properties depend on the surface structure, temperature, and wavelength of radiation. Some key radiative properties are:
- Emissivity – efficiency of emitting thermal radiation
- Reflectivity – fraction of incident radiation reflected
- Transmissivity – fraction of incident radiation transmitted through a material
- Absorptivity – fraction of incident radiation absorbed
Materials like metals are good reflectors and poor emitters/absorbers. Non-metals like glass are often good transmitters. Black surfaces are excellent absorbers and emitters. Engineers take advantage of these properties in applications like solar water heating, satellite thermal control, and infrared sensing.
Advection
Advection is the transfer of heat by the flow of a fluid. Specifically, it refers to the transport of heat by the large-scale motion of currents in a fluid like air or water. As the fluid flows and mixes, it carries heat along with it, transferring thermal energy from one location to another. Advection is one of the main mechanisms of heat transfer in liquids and gases.
Some examples of heat transfer by advection include:
- Winds carrying heat from warm areas to cooler areas
- Ocean currents redistributing heat around the globe
- Hot water rising and cooler water sinking in a pot of boiling water
- Heat being dispersed through the flow of air from a hairdryer or fan
- Heat being carried along pipes by the flow of hot or cold water
Advection plays a key role in heat transfer and energy transport in many natural and engineering systems. It allows large amounts of thermal energy to be rapidly transferred over great distances by the bulk motion of fluids, enabling the redistribution of heat on scales ranging from your home’s heating system to global climate patterns.
Diffusion
Diffusion is the movement of heat from the hotter parts of a substance to the cooler parts. It occurs at a molecular level without any noticeable motion of matter in the space. Heat is transferred between neighboring molecules by direct particle interactions. The warmer particles have higher kinetic energy, which allows them to collide with neighboring cooler particles and transfer some of their energy. Over time, this results in a more uniform thermal energy distribution.
Diffusion plays an important role in heat transfer, especially in solids and liquids. It enables thermal conductivity as heat diffuses through the material from higher to lower energy regions. Some examples of diffusion in heat transfer include:
- Heat spreading through the metal walls of a cooking pot on the stove.
- Warmth from a heating pad gradually transferring into the surrounding body tissue.
- Temperature equalizing in a glass of water that starts with hot water on one side and cold water on the other.
- The handle of a cast iron skillet heating up as heat from the cooking surface is conducted through the metal.
Without diffusion, many materials would not be able to conduct heat effectively. The diffusive motion spreads thermal energy at a molecular level, enabling heat conduction through the material.
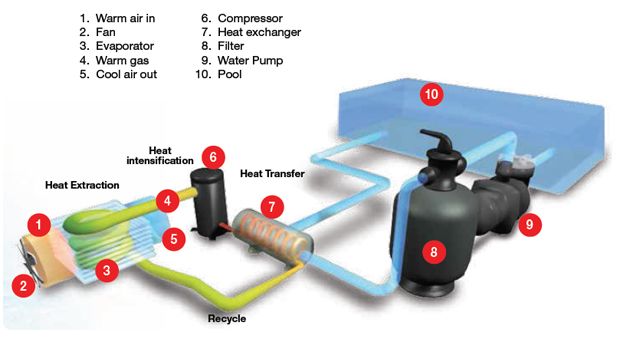
Heat Exchanger
A heat exchanger is a device designed to efficiently transfer heat between two or more fluids at different temperatures. The fluids can be single or two-phase and are separated by a solid wall, so they never mix. Heat exchangers are commonly used in heating, refrigeration, air conditioning, power plants, petrochemical plants, petroleum refineries, and natural gas processing.
Some common examples of heat exchanger applications include:
- In automobiles, the radiator transfers heat from the hot coolant that flows through the engine block to the air blown through the radiator fins.
- In HVAC systems, the evaporator coils transfer heat from the air to the refrigerant to provide cooling for the enclosed space.
- In power plants, steam generators (boilers) transfer heat from hot gases to water to produce steam for the turbines. Feedwater heaters heat up water before it enters the boiler using extracted steam.
- In oil refineries, heat exchangers are used extensively to heat and cool fluids at various steps of the refining process.
- In chemical plants, heat exchangers heat or cool process fluids, condense vapors, and recover heat between process streams.
Heat exchangers provide more efficient heat transfer than simple heat transmission through a wall. Their performance can be optimized by maximizing the surface area of the wall between the fluids and encouraging turbulent mixing in the fluids. Common designs include shell-and-tube, plate, and fin tube heat exchangers.
Thermal Resistance
Thermal resistance is a measure of a material’s ability to resist heat flow. It indicates how much temperature difference is required to induce a unit of heat flux through a structure. Materials with low thermal resistance allow heat to flow through them easily, while materials with high thermal resistance impede heat transfer.
The thermal resistance of a material depends on its thickness and thermal conductivity. Thermal conductivity (k) measures a material’s ability to conduct heat. Materials like metals have high thermal conductivity, allowing heat to pass through them readily. Insulators like plastic foams have low thermal conductivity, which resists heat flow.
Thicker materials have higher thermal resistance than thinner ones made of the same material. Heat must pass through more material, which increases resistance to flow.
Materials with low thermal resistance, like aluminum or copper, are used when heat needs to be transferred efficiently. Fins on heat sinks are made of metals to allow heat to dissipate rapidly from hot components. High thermal resistance materials like plastic and rubber are used for insulation to impede heat loss. Special insulations like fiberglass batts and rigid foam boards have extremely low thermal conductivity, providing excellent thermal resistance for buildings and refrigerators.
Understanding thermal resistance allows appropriate materials to be selected to control heat transfer for different applications. Low resistance enables efficient cooling of electronics, while high resistance prevents unwanted gain or loss of heat in vehicles, appliances, and structures. Tailoring thermal resistance through material choice and thickness is key for designing effective insulation systems and heat transfer devices.
Heat Sink
A heat sink is a passive component that absorbs and dissipates heat from another device via thermal contact. Heat sinks are commonly used in computers and other electronics to cool components and prevent overheating. Some key aspects of heat sinks include:
- Made of materials with high thermal conductivity like aluminum, copper or steel to readily conduct heat away from the heat source.
- Designed with fins or ridges to increase the surface area that contacts the surrounding air, improving heat dissipation.
- May use a fan directed over the fins to facilitate forced air convection and enhance heat dissipation.
Common examples of heat sink applications include:
- CPU and GPU heat sinks in computers to dissipate heat generated by the processor.
- Heat sinks on power amplifiers or transistors to dissipate heat and prevent component failure.
- Heat spreaders on RAM modules to distribute localized heat over a larger area.
- Heat sinks on hot objects like stoves, ovens or fireplaces to protect surrounding surfaces.
Heat sinks play a crucial role in temperature regulation and thermal management across electronics and industrial applications. Proper heat sink design, material selection and airflow optimization are key to maintaining safe operating temperatures.
Conclusion
In summary, there are several key types of heat transfer that describe how thermal energy moves from one place to another. The main modes of heat transfer are conduction, convection, and radiation.
Conduction refers to the transfer of heat between substances that are in direct contact with each other, such as a pot on a stove. The better the conducting material, the faster heat will be transferred through it.
Convection is the movement of heat through fluids, either gases or liquids. Hotter, less dense material rises, while colder denser material sinks, causing circulation of heat.
Radiation is the emission of electromagnetic waves from all matter above absolute zero. It can transfer heat without relying on particle collision.
Other specialized types of heat transfer include advection, the transfer of heat by the bulk motion of a fluid, diffusion, the spread of heat through random molecular motion, and several modes of engineered heat transfer used in heat exchangers.
By understanding the various mechanisms of heat transfer, we can better design systems to take advantage of or combat the flow of thermal energy. This allows us to effectively heat and cool objects ranging from electronics to buildings.