What Is The Formula For Heat Energy And Work?
Heat energy and work are two important concepts in thermodynamics that are often related through mathematical formulas. The goal of this article is to clearly define what heat energy and work represent in physics and derive the formula that allows calculation of one quantity using the other. With a solid understanding of the relationship between heat transfer and work, we can apply these principles to real-world thermodynamic systems and processes.
Definitions of Heat Energy and Work
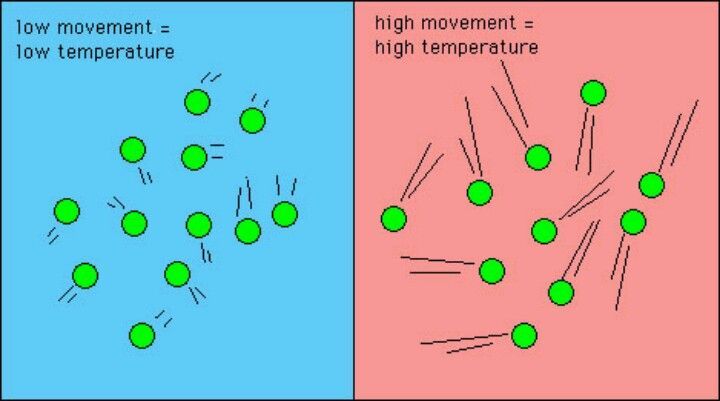
Heat energy is the transfer of thermal energy between systems due to a temperature difference. It refers to the amount of energy that is transferred from a higher temperature system to a lower temperature system. The SI unit for heat energy is the joule (J). The calorie (cal) is also commonly used.
Work is the transfer of energy resulting from a force acting on an object through a displacement. Work occurs when an object is moved against an opposing force. The SI unit for work is the joule (J).
Heat and work are related, but distinct forms of energy transfer. Heat involves random molecular motion being transferred whereas work involves organized motion and displacement against a force.
The First Law of Thermodynamics
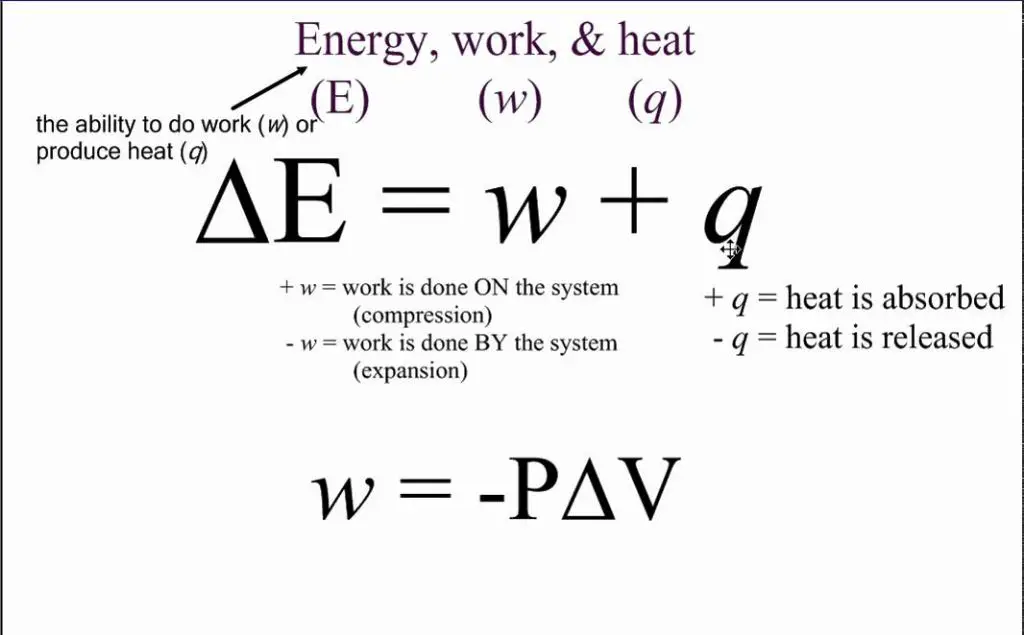
The first law of thermodynamics is a fundamental physical law that states that the change in internal energy of a closed thermodynamic system is equal to the difference between the heat supplied to the system and the work done by the system on its surroundings. Symbolically, this can be expressed mathematically as:
ΔU = Q – W
Where ΔU is the change in internal energy, Q is the heat added to the system, and W is the work done by the system. This first law establishes that energy is conserved in thermodynamic processes. The internal energy U of a closed system can only be changed by transferring energy as heat Q or doing work W. This law connects the concepts of heat and work, showing that both can be viewed as ways to transfer energy into or out of a system.
The first law provides the foundation for the science of thermodynamics. It implies that perpetual motion machines are impossible, since such imaginary machines would violate the law of conservation of energy. By relating heat and work, it also sets the stage for defining the concept of entropy and for analyzing thermodynamic cycles.
The Formula Relating Heat and Work
The relationship between heat energy (Q) and work (W) is given by the following formula:
Q = ΔU + W
Where:
- Q is the amount of heat energy transferred.
- ΔU is the change in internal energy of the system.
- W is the amount of work done on or by the system.
This is known as the first law of thermodynamics. It states that the change in internal energy of a closed system is equal to the amount of heat supplied to the system minus the amount of work done by the system on its surroundings.
In this formula:
- If Q is positive, then heat energy is being transferred to the system.
- If W is positive, then work is being done on the system.
- If Q or W are negative, then heat or work is being transferred out of the system.
This fundamental relationship allows us to calculate changes in the internal energy of a system based on the heat and work interactions it undergoes with its surroundings. It is a key tool for analyzing thermodynamic processes.
Heat as Transfer of Energy
Heat is a form of energy transfer between objects or systems due to temperature difference. Heat always flows spontaneously from a hotter object to a colder object until they reach thermal equilibrium. This occurs because higher temperature means greater molecular motion and kinetic energy, so heat represents the transfer of this kinetic energy from more energetic to less energetic molecules.
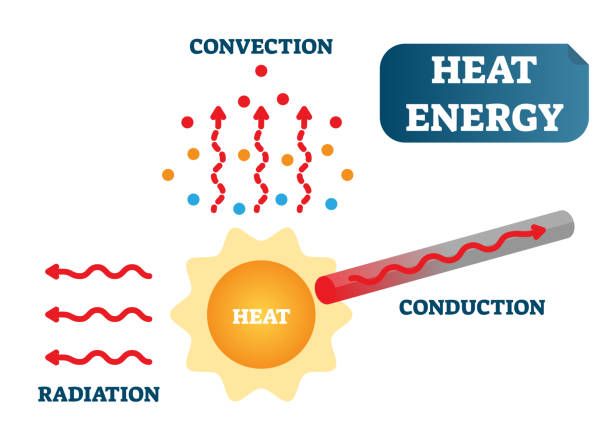
There are three main mechanisms of heat transfer:
- Conduction – Heat transfer through direct contact between materials. The more energetic vibrating atoms directly collide with the less energetic atoms, transferring kinetic energy.
- Convection – Heat transfer via the circulation or movement of liquids or gases. Heated fluid rises while cooler fluid sinks, transporting thermal energy by bulk flow.
- Radiation – Heat transfer via electromagnetic waves. All objects emit thermal radiation related to their temperature, allowing heat to be transferred across space without the need for direct contact or fluid transport.
Understanding how heat energy flows is crucial for designing thermal systems like engines, refrigerators, buildings, and more. The spontaneous flow of heat is the driving force behind many essential technologies and processes. Quantifying and controlling heat transfer enables modern heating, cooling, power generation, and manufacturing.
Work as Transfer of Energy
Work in thermodynamics refers to the transfer of energy between a system and its surroundings due to some process or change in the system. For example, when a gas expands against a movable piston, it does work by exerting a force over a distance on the piston. This transfers energy from the gas to the piston, changing the energy of both the system (gas) and surroundings (piston).
More formally, thermodynamic work is defined as force multiplied by displacement. If a force F acts over a displacement d, it does an amount of work W given by:
W = F*d
The force and displacement must be in the same direction for positive work. If they are in opposite directions, the work will be negative. This sign convention indicates whether net energy is transferred into (positive work) or out of (negative work) the system.
For example, when compressing a gas in a cylinder, work is done on the gas by the piston, transferring energy into the gas and increasing its internal energy. The gas exerts a force in the direction opposing the piston’s motion, so the product F*d is negative and the work done on the gas is negative.
In summary, thermodynamic work represents the energy transfer between a system and its surroundings due to some mechanical process. Calculating work as force times displacement reveals whether the net energy transfer is into or out of the system.
Sign Conventions for Heat and Work
When using the formula Q = ΔU + W, it’s important to pay attention to the sign conventions for heat (Q) and work (W) based on whether energy is entering or leaving the system.
For heat:
- If heat is entering the system, Q is positive.
- If heat is leaving the system, Q is negative.
For example, if a burner is heating up a pot of water, heat is entering the water (the system), so Q is positive.
For work:
For example, if the water pushes up on the pot lid, doing work to lift the lid, W is positive. But if gravity pushes down on the water, doing work by compressing it, W is negative.
So in summary, heat entering the system and work done by the system are positive Q and W. Heat leaving the system and work done on the system by surroundings are negative Q and W.
Examples and Calculations
We can use the formula Q = ΔU + W to calculate heat and work in different scenarios. Let’s look at some example problems:
1) A gas expands against a constant external pressure of 1 atm, doing 40 J of work. The internal energy of the gas decreases by 25 J. Calculate the heat flow.
Plugging the values into the equation:
Q = ΔU + W
Q = -25 J + 40 J
Q = 15 J
The heat flow is 15 J.
2) A gas is compressed, decreasing its volume. In this process, the system loses 50 J of heat to the surroundings and has 15 J of work done on it. What is the change in internal energy?
Q = ΔU + W
-50 J = ΔU + (-15 J)
ΔU = -50 J – (-15 J)
ΔU = -35 J
The change in internal energy is -35 J.
In the first example, work is positive since the gas expands against an external pressure. In the second, work is negative since work is done on the gas to compress it.
This demonstrates how to apply the heat and work formula Q = ΔU + W to different thermodynamic processes with proper sign conventions.
Applications
The heat-work formula has many important applications in thermodynamic systems like engines and refrigerators. Here are some examples:
In a car engine, chemical energy from burning fuel is converted into heat energy, which is then partially converted into useful work to move the pistons. The leftover heat must be dissipated through the radiator and exhaust system. Engineers apply the heat-work equation to maximize the work extracted and design efficient cooling systems.
In a refrigerator, work is done by the compressor to remove heat from the inside and pump it to the outside. The heat-work formula governs the amount of work needed to remove a certain quantity of heat in order to keep the refrigerator interior cold. Refrigerators are designed to move heat as efficiently as possible.
Power plants also rely on the relationship between heat and work. Heat is generated from burning fuel and used to boil water into steam that drives turbine generators. The difference between the heat input and work output represents the wasted heat that must be disposed of through cooling towers. Understanding these thermodynamic principles allows power plant operators to maximize electricity generation.
The heat-work formula is a fundamental relationship applied in the design and analysis of heat engines, refrigerators, and many other important thermodynamic systems. Mastering this concept is crucial for mechanical and aerospace engineers working on engine design, as well as physicists and chemists studying thermodynamic processes.
Summary
In summary, heat energy and work are related through the first law of thermodynamics, which states that the change in internal energy of a system is equal to the amount of heat added to the system minus the work done by the system. The mathematical formula relating heat and work is:
ΔU = Q – W
Where ΔU is the change in internal energy of the system, Q is the amount of heat added to the system, and W is the work done by the system. This shows that heat added and work done are two ways of transferring energy into or out of a system and causing its internal energy to change. Heat is energy transfer due solely to temperature difference, while work is energy transfer driven by a force exerted through a distance. Understanding the relationship between heat and work through this formula is key for analyzing many thermodynamic processes.
To recap, the first law of thermodynamics links the concepts of heat energy and work through a simple but powerful equation. This reveals the connection between these two methods of energy transfer and how they both impact the internal energy of a system.