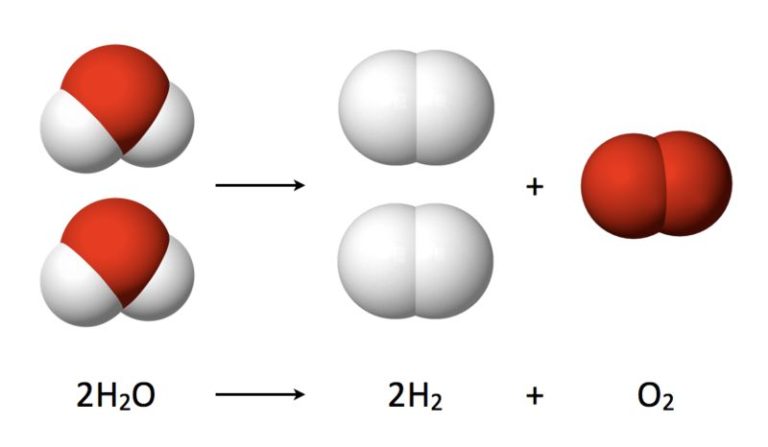
What Is The Energy That Causes Change Or Movement?
Introduction
Energy is defined as the ability to do work or cause change. It is the capacity to move or transform matter. Energy exists in many different forms such as heat, light, motion, or electricity. It enables all motion, life, and processes in the universe.
Energy can be converted or transformed from one type into another. For example, a battery converts chemical energy into electrical energy to power a flashlight. The flashlight bulb then converts that electrical energy into light and heat. The law of conservation of energy states that energy cannot be created or destroyed, but only transformed from one form into another.
Understanding the different forms of energy and how they can change is key to harnessing energy for human purposes. Energy is involved in all activities and allows living things to grow, move, and function. Learning to utilize various energy forms has enabled society to advance technologically.
Potential Energy
Potential energy is the energy stored in an object due to its position or arrangement. There are several types of potential energy including gravitational potential energy, elastic potential energy, and chemical potential energy.
Gravitational potential energy is energy stored in an object due to its height above the ground. For example, a ball held at a height above the ground has gravitational potential energy due to the pull of gravity on the ball. The higher the ball is held, the more gravitational potential energy it possesses.
Elastic potential energy is energy stored in elastic materials that are deformed or stretched. For example, a stretched rubber band has elastic potential energy. The more the rubber band is stretched, the more elastic potential energy it contains.
Chemical potential energy is energy stored in the bonds between atoms and molecules. This energy can be released in chemical reactions. For example, food contains chemical potential energy that is released when the food is digested.
In summary, potential energy exists as stored energy in an object due to its position or arrangement, such as height, stretch, or chemical composition. This energy has the potential to be converted into kinetic energy or used to do work.
Kinetic Energy
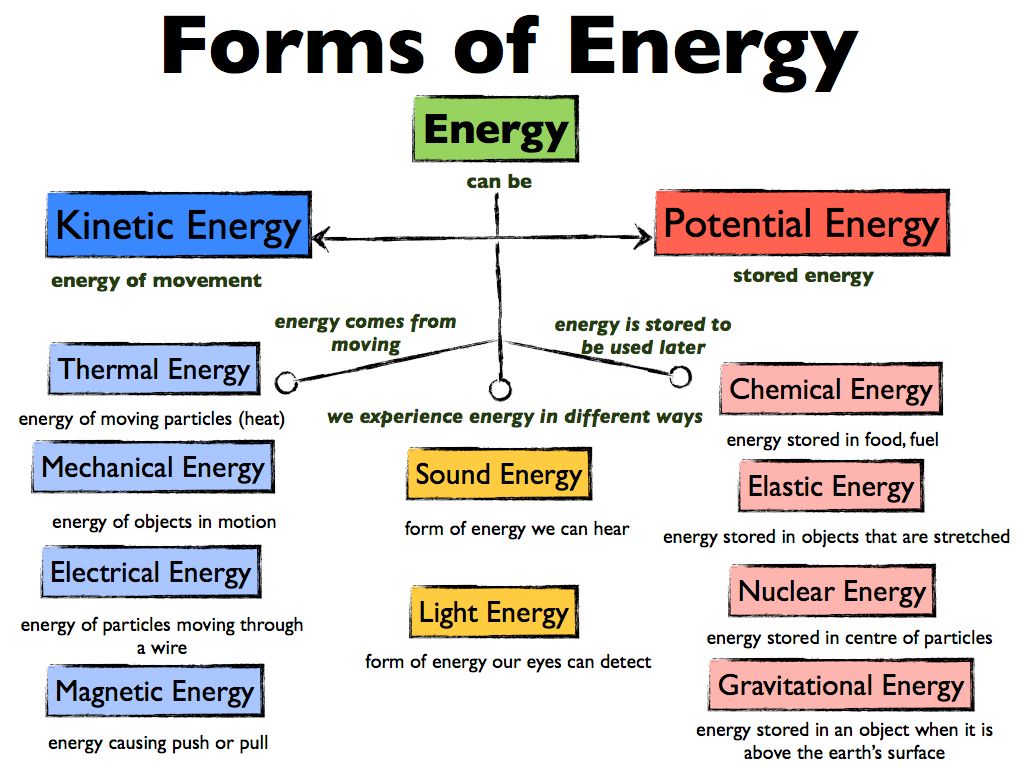
Kinetic energy is the energy associated with motion. An object that is in motion, such as a ball rolling down a hill or a car driving down the road, has kinetic energy. The faster the object is moving, the more kinetic energy it possesses.
The amount of kinetic energy that an object has depends on two variables:
- The object’s mass – A more massive object moving at the same speed as a less massive object will have more kinetic energy.
- The object’s velocity – The faster the object is moving, the more kinetic energy it has. Velocity refers to the speed and direction of motion.
Kinetic energy can be transferred between objects. For example, when one billiard ball strikes another, some of the kinetic energy from the moving ball is transferred to the stationary ball, causing it to move. The total kinetic energy of the two objects remains the same before and after the collision (assuming no energy is lost to heat or sound).
Examples of kinetic energy in everyday life include a person walking or running, objects falling under gravity, and any moving vehicles. The movement of molecules and atoms also contains kinetic energy. Unlike potential energy, kinetic energy is directly observable as motion.
Mechanical Energy
Mechanical energy is the sum of potential energy and kinetic energy in a system. It refers to the energy associated with the motion and position of an object. Potential energy depends on the position or configuration of an object and is the stored energy it has due to its position. For example, a ball held at a height above the ground has potential energy due to gravity. Kinetic energy is the energy an object has due to its motion. For example, a rolling ball has kinetic energy. When the ball is released, its potential energy is converted into kinetic energy as it falls and gains speed. The total mechanical energy remains constant in isolated systems without friction or other non-conservative forces. This is known as the conservation of mechanical energy and is a fundamental concept in physics.
Thermal Energy
Thermal energy refers to the energy associated with the microscopic kinetic energy of atoms and molecules. It is directly proportional to the temperature of matter – the higher the temperature, the greater the thermal energy. This energy manifests as the random motion and vibrations of particles that make up matter. The faster the particles move and vibrate, the higher the thermal energy.
Heat is the transfer of thermal energy between objects or systems due to their temperature difference. Heat always flows spontaneously from a hotter to a colder body. Thermal energy can do work when it is converted into mechanical energy. For example, the burning of fuel in an engine converts thermal energy into mechanical work. Thermal energy is also responsible for changes in the states of matter, like melting of ice into water, or boiling of water into steam.
Because thermal energy is associated with random molecular motion, it is considered a less ordered or less usable form of energy. When thermal energy is transformed into work, some amount of energy is always wasted or lost due to irreversibilities. This loss manifests as an increase in entropy or disorder in the universe. The Second Law of Thermodynamics formalizes this notion.
Radiant Energy
Radiant energy is a form of energy that is transferred by electromagnetic waves. It does not rely on direct contact for energy transfer and can travel long distances through space. The term “radiant” refers to the way this energy radiates out from its source. Some key facts about radiant energy:
- Radiant energy is carried by electromagnetic waves which are oscillating electric and magnetic fields.
- It includes visible light that we can detect with our eyes, as well as other forms of electromagnetic radiation that are invisible to us like infrared, ultraviolet, radio waves, X-rays, and gamma rays.
- The sun is a major source of radiant energy. The sunlight that reaches Earth contains a spectrum of radiant energy including ultraviolet rays, visible light, and infrared waves.
- Other sources of radiant energy include fire, radiant heaters, radio transmitters, and higher frequency radiation from X-ray machines or radioactive materials.
- Radiant energy travels at the speed of light which is approximately 186,000 miles per second.
- It can propagate through empty space or some mediums like air and glass. Other materials like metals will reflect or absorb radiant energy.
- The intensity of radiant energy decreases proportionally to the square of the distance from the source, following the inverse square law.
Nuclear Energy
Nuclear energy refers to the energy stored in the nucleus of an atom. Atoms are made up of neutrons, protons, and electrons. The neutrons and protons are clustered together in the nucleus, which is held together by a strong nuclear force. Nuclei that are stable do not release energy, but unstable or radioactive nuclei release energy as they decay or break apart into more stable nuclei.
There are two main kinds of nuclear reactions that can release energy – nuclear fission and nuclear fusion. In nuclear fission, a heavy, unstable nucleus splits into two or more lighter nuclei, releasing energy in the process. This energy can be harnessed to generate electricity. Fission reactions power nuclear power plants and also atomic bombs. In nuclear fusion, two light nuclei fuse together to form a heavier stable nucleus, releasing an enormous amount of energy. This is the process that powers the sun and other stars. Fusion reactions release even more energy than fission but controlling fusion energy on Earth for power generation remains a key scientific and engineering challenge.
Overall, nuclear energy represents an extremely concentrated source of power. The binding energy stored in nuclei can produce millions of times more energy per unit mass than chemical energy sources like fossil fuels. However, there are risks associated with nuclear reactions, including radiation exposure and nuclear proliferation. Careful design and oversight are required to safely utilize nuclear energy.
Electrical Energy
Electrical energy refers to energy that comes from the movement of electrons. Electrons have a negative charge, and when they move through a conductor, they generate electricity. This electricity can then be harnessed and used to power numerous devices and equipment. Here are some key things to know about electrical energy:
Electrical energy is produced when electrons flow through a conductor. The electrons are surrounded by the atoms that make up the conductive material. As the electrons move, they bump into the atoms and transfer some of their energy to them. This creates an electric current that flows through the conductor.
Common sources that produce electrical energy include batteries, generators, and electrochemical cells. In a battery, chemical reactions push electrons from one terminal to another, generating an electrical current. Generators use mechanical energy like wind or steam to spin copper coils within a magnetic field, moving electrons and producing electricity.
Electrical energy is extremely versatile and powers a wide array of devices. Anything that is plugged into an outlet, powered by a battery, or connected to a generator utilizes electrical energy. From lights, appliances, and electric vehicles to computers, cell phones, and medical devices – they all rely on the movement of electrons to operate.
Electrical energy can also easily be converted into other forms of energy. For example, a light bulb converts electrical energy into radiant energy in the form of light and heat. An electric motor converts electrical energy into kinetic energy to drive machinery. Electrical energy drives modern society and is integral to almost every aspect of our daily lives.
Chemical Energy
Chemical energy is the energy stored in the bonds between atoms and molecules. It is released when chemical bonds are formed and broken in chemical reactions.
For example, the food we eat contains chemical energy that was originally captured from sunlight by plants through photosynthesis. When food is digested, chemical bonds are broken and that energy is released and made usable by our cells. Another example is the chemical energy stored in batteries. This energy is released as electricity when chemical reactions take place between the chemicals inside the battery.
Fossil fuels like coal, oil and natural gas also contain tremendous amounts of chemical energy from the remains of ancient plants and animals. When burned, their chemical bonds break down, releasing heat and energy that can be used to generate electricity or power engines. The chemical energy in fossil fuels is responsible for most of the world’s power generation today.
Chemical energy drives many important biological and geological processes on Earth. Our bodies rely on converting chemical energy from food into thermal and mechanical energy to survive. The convection of magma in the Earth’s mantle is driven by the release of chemical energy as well. Understanding chemical energy is key to harnessing its potential.
Conservation of Energy
Energy cannot be created or destroyed, only transformed from one form to another. This is the first law of thermodynamics and a fundamental principle of physics. It states that the total energy in a closed system always remains constant, though energy transformations continuously occur within the system.
A closed system refers to a region of space that does not exchange matter with its surroundings and is isolated from external forces. The total energy within such a system is conserved at a fixed value. For example, a bouncing ball on a table is a closed system. When the ball falls, its potential energy transforms into kinetic energy, but the total mechanical energy of the system remains unchanged. As the ball bounces back up, its kinetic energy transforms back into potential energy. The energy changes form, but the total amount is conserved.
This principle of conservation of energy applies universally across all physical interactions. Whether it is chemical reactions, phase changes, nuclear processes, or any form of work and heat transfer, the total energy remains the same. Energy is never lost but only converted from one type to another. For example, chemical energy in gasoline is converted to kinetic energy in a car engine, while solar radiant energy is converted to electrical energy in solar panels. The combined energy, however, is always conserved.
The law of conservation of energy is a fundamental tenet of physics and explains that energy transformations drive all the dynamic processes we observe in nature and technology. Though energy converts from one form to another, the total energy in a closed system remains fixed.