What Is The Difference And Similarity Between Work And Energy?
Work and energy are two fundamental concepts in physics that describe how forces affect the motion of objects. Understanding the difference and similarity between work and energy provides insight into how the physical world operates. While work and energy may seem distinct at first glance, they are intimately related, and one can often be converted into the other. Grasping this relationship allows us to better analyze mechanical systems and predict their behavior. The interplay between work and energy also gives rise to the important principle of conservation of energy, which states that energy can never be created or destroyed, only transformed from one form to another. In this article, we will define work and energy, explain their units of measurement, discuss the key relationships that connect them, and provide illustrative examples to demonstrate their relevance in real-world systems.
Definitions of Work and Energy
Work and energy are two fundamentally important concepts in physics.
Work is defined as force applied over a distance. The mathematical definition is:
Work = Force x Distance
Where force is measured in Newtons (N) and distance is measured in meters (m). For example, lifting a 10 kg object 1 meter vertically results in work equal to:
Work = Force x Distance
= 10 kg x 9.81 m/s2 x 1 m
= 98.1 Nm (joules)
Energy is defined as the ability to do work. There are many forms of energy such as kinetic, potential, thermal, chemical, etc. The two most relevant forms in physics are kinetic and potential energy.
Kinetic energy is energy associated with motion. The mathematical definition is:
Kinetic Energy = 1/2 x mass x velocity2
Where mass is in kg and velocity is in m/s. For example, a 2 kg object moving at 3 m/s has a kinetic energy of:
Kinetic Energy = 0.5 x 2 kg x (3 m/s)2
= 9 Joules
Potential energy is stored energy that depends on an object’s position or configuration. For example, a ball held at a height above the ground has gravitational potential energy due to the earth’s gravity pulling it downwards. The mathematical definition is:
Gravitational Potential Energy = mass x gravity x height
Where mass is in kg, gravity is 9.81 m/s2, and height is in meters. For example, lifting a 5 kg object to a height of 2 meters results in a gravitational potential energy of:
Gravitational Potential Energy = 5 kg x 9.81 m/s2 x 2 m
= 98.1 Joules
Units of Measurement
Both work and energy are measured in the same units in the SI (international system) of measurement. The unit for work and energy is the joule, represented by the symbol J.
A joule is defined as the work done by a force of one newton when its point of application moves one meter in the direction of action of the force. One joule is equivalent to the energy required to produce one watt of power for one second.
Some examples of joule values:
- 1 J = The energy required to lift an apple 1 meter against gravity
- 4.2 J = The kinetic energy of a tennis ball moving at 30 mph
- 1055 J = The amount of potential energy stored in a 50g mass lifted 2m above the ground
So in summary, the standard metric unit for both work and energy is the joule (J). This allows the two quantities to be directly compared and mathematically related.
Relationship Between Work and Energy
There is a direct relationship between the work done on an object and its change in energy. When a force acts on an object and causes it to move, work is done on the object. This work causes a change in the object’s kinetic energy if it is moving, or potential energy if it is stationary.
The work done on an object is equal to the change in its energy. This is described by the work-energy theorem, which states that the net work done on an object equals its change in kinetic energy. This is represented by the equation:
W = ΔKE
Where W is the net work done on the object and ΔKE is its change in kinetic energy. The net work increases the object’s energy, either kinetic or potential. This demonstrates the direct transfer between work and energy. The amount of work done is directly proportional to the resulting change in the object’s energy.
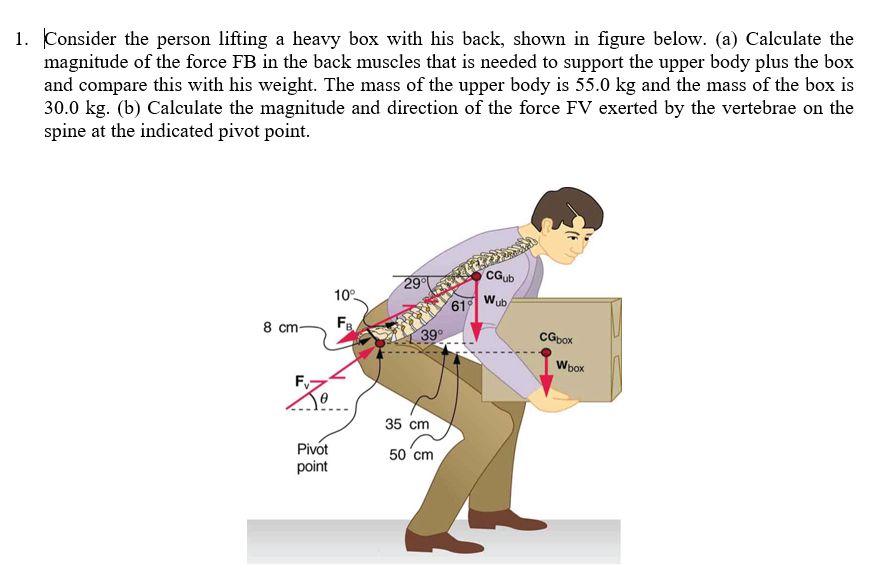
This relationship is an important concept in physics and engineering. It shows that work and energy are intrinsically linked. Work done on an object must transfer into energy, ensuring the conservation of energy in a system. This principle has many useful applications in analyzing mechanical systems and processes.
Examples
Here are some examples that demonstrate how doing work leads to a change in energy:
Lifting a heavy box: When you lift a heavy box from the ground up onto a table, you are doing work to move the box upward. This work requires your muscles to exert force over a distance as you lift. The chemical potential energy stored in your body from food is converted into kinetic energy and gravitational potential energy as you lift the box upward. The higher the box is lifted, the more gravitational potential energy it gains.
Stretching a spring: When you stretch or compress a spring, you are doing work to change its shape. This work goes into storing elastic potential energy in the spring. The more the spring is stretched or compressed, the more elastic potential energy gets stored. When released, this energy is converted into kinetic energy as the spring returns to its original shape.
Friction when rubbing hands: The act of rubbing your hands together does work by overcoming frictional forces between the skin surfaces. This work causes an increase in the thermal energy and temperature of your hands by converting mechanical energy into heat. The faster you rub your hands, the more work is done and the more heat is produced.
These examples demonstrate how in physics, work is directly related to changes in the energy of a system. Doing work to move an object, deform an object, or overcome friction leads to energy being shifted between different stores of potential energy, kinetic energy, and thermal energy.
Conservation of Energy
One of the most fundamental laws of physics is the law of conservation of energy. This law states that in an isolated system, the total amount of energy remains constant. Energy cannot be created or destroyed, only converted from one form to another.
For example, when a ball falls to the ground, its potential energy gets converted to kinetic energy as it accelerates due to gravity. The kinetic energy gets converted to heat and sound energy when the ball hits the ground. While the forms of energy change, the total amount of energy in the isolated system (the falling ball) remains the same.
The law of conservation of energy is directly related to work and energy. When positive work is done on an object, it gains energy. When negative work is done, the object loses energy. However, if we take into account the energy gained or lost by the surroundings that are doing the work, the total energy is conserved. The energy is simply transferred from one system to another.
Understanding the conservation of energy is crucial for analyzing mechanical, electrical, and many other systems. It allows us to track where energy is transferred and converted. While energy changes form, the total quantity always remains the same.
Differences Between Work and Energy
While work and energy are related concepts in physics, there are some key differences between them:
Nature: Work is a process whereas energy is a property of a system. Work is done when a force displaces an object, energy exists within an object or system.
Units: Work is measured in joules (J) in the SI system, whereas energy is measured in joules (J) for all forms of energy. Work has the same units as energy, but it is not a form of energy in itself.
Scalar vs Vector: Work is a scalar quantity with magnitude only, while forms of energy like kinetic energy and potential energy are scalar quantities. However, energy transfers can be vector quantities.
Point of Application: Work is done by an applied force, so it depends on the point of application of the force. The amount of energy possessed by a body is independent of the coordinate system or point of application.
Calculations: Work done is calculated as force x displacement. Energy is calculated by different equations based on the form (kinetic, potential, etc). The work-energy theorem relates work done to the change in kinetic energy.
SI Units: As mentioned earlier, work and energy both use the SI unit of joules (J). However work can also be expressed in derived units like newton-meters (N⋅m) while energy cannot.
Mechanisms: There are different mechanisms by which energy can be transferred between objects and transformed between forms. Work is specifically the transfer of energy through an applied force displacing an object.
Similarities
Even though work and energy are different concepts, there are some key similarities between them that are worth noting:
Units of measurement – Work and energy are both measured in the same units, such as joules (J) in the SI system. The fact that they share the same units gives an indication that they are related quantities.
Transference – Energy can be transferred from one system to another as work. For example, when you lift a book from the floor to a table, your body’s chemical energy is transferred to the book in the form of kinetic energy and potential energy. The work done on the book is equal to the energy transferred.
Conservation – They both follow the law of conservation of energy, which states that energy can neither be created nor destroyed in an isolated system. The total amount of work done on a system will result in an equal amount of change in the system’s total energy.
Calculations – Calculations involving work and energy are directly related through their mathematical formulas. The work done on an object can be calculated using its change in energy.
So in summary, while work and energy are distinct concepts, they share important similarities in their units, transferability, conservation, and mathematical relationship that connects the two quantities.
Applications
Work and energy concepts are applied in many real-world scenarios. Here are some examples:
In engineering, work and energy calculations are used extensively in the design and analysis of machines, structures, and systems. For example, calculating the kinetic and potential energy of objects allows engineers to predict the forces and motion involved in their operation.
In physics, work-energy calculations help explain phenomena and solve problems. For instance, the work-energy theorem can be used to determine the speed of an object after it has traveled a certain distance under the influence of a force.
In sports science, analysis of athletic performance relies on energy and work principles. The metabolic energy expended by an athlete can be estimated based on their motion, and improvements in technique can optimize the work output.
In biology, living organisms require energy intake to perform mechanical work. The efficiency of that conversion from food energy to useful work plays a role in health, fitness and functioning.
In economics, work contributes to the productivity and GDP of nations. Energy resources like oil, gas and electricity impact the economic growth, standard of living and geopolitics between countries.
In ecology, tracking energy transfers between different components of ecosystems provides insights into food chains, predator-prey relations, and impact of human activities.
Conclusion
In summary, while work and energy are closely related concepts in physics, there are some key differences between them. Work involves force applied over a distance, while energy is the capacity to do work. Work is a process, whereas energy is a property of objects.
However, work and energy are connected by the principle of conservation of energy. The work done on an object transfers energy to that object. The total mechanical energy in a closed system remains constant, even as energy transforms between potential and kinetic forms.
Understanding the relationship between work and energy is crucial for analyzing mechanical systems. Engineers apply these principles to improve efficiency in machinery and technology. Knowing how to calculate work and energy also provides insight into the behavior of objects in motion.
While work and energy have distinct meanings, they are fundamentally linked. Grasping both concepts and their connections is key to mastering physics and engineering applications. With an overview of their differences and similarities, we gain a deeper understanding of how energy flows in the physical world.




