What Is The Definition Of Chemical Potential Energy In Chemistry?
Chemical potential energy is the potential energy stored in the chemical bonds between atoms within a molecule or compound. It refers to the energy released or absorbed during a chemical reaction or phase transition.
Understanding chemical potential energy is fundamental to chemistry and physics, as it allows us to predict whether a reaction will occur spontaneously and how much energy will be released. It is critical for designing batteries, fuels, explosives, and other energy technologies.
In this article, we will define chemical potential energy more formally, discuss different types of chemical bonds and how they store energy, explain how to calculate energy changes in chemical reactions, and overview some key applications like batteries and metabolism.
Definition
Chemical potential energy is the potential energy stored in chemical bonds between atoms and molecules. It is the energy that can be released or absorbed during a chemical reaction, when chemical bonds are broken and new chemical bonds are formed. The chemical potential energy of a system depends on the atomic structure, molecular geometry, and bonding pattern.
For example, the bonds between carbon and hydrogen atoms in hydrocarbon fuels like gasoline contain substantial chemical potential energy. This energy can be released during combustion reactions when new bonds are formed between the carbon, hydrogen, and oxygen atoms in the reactants. The release of this stored chemical energy is what powers many combustion engines.
In an exothermic chemical reaction, the total chemical potential energy of the products is less than that of the reactants. This releases energy to the surroundings as heat and light. In an endothermic reaction, the products have greater chemical potential energy than the reactants, so energy must be absorbed from the surroundings for the reaction to proceed.
Types of Chemical Bonds
There are three main types of chemical bonds that are capable of storing potential energy:
Ionic bonds form between metal and nonmetal ions with opposite charges. The electrostatic attraction between the positive and negative ions gives ionic compounds their lattice energy. This energy must be overcome to break the ionic bonds during melting or dissolving the compound.
Covalent bonds form when atoms share pairs of electrons. Energy is required to overcome the electron-pair sharing in order to break covalent bonds. The strength of covalent bonds determines the stability and potential energy stored in molecules.
Hydrogen bonds occur between molecules when a hydrogen atom bound to a highly electronegative atom forms an electrostatic link with another electronegative atom nearby. Although weaker than ionic or covalent bonds, hydrogen bonds store enough energy that must be broken for chemical reactions or changes of state to occur.
Energy Storage
When atoms bond together to form a molecule or compound, they exist at a lower potential energy level compared to existing as separate atoms. This means that energy is stored in the chemical bonds holding the atoms together.
For example, when two hydrogen atoms bond with one oxygen atom to form a water molecule (H2O), energy is released as the bonds are formed. The water molecule is at a lower energy state than the separated hydrogen and oxygen atoms. This released energy from bond formation is stored as chemical potential energy in the water molecule.
To break the bonds in water and separate the molecule back into individual hydrogen and oxygen atoms, energy must be added to the system. Breaking chemical bonds requires energy input to overcome the attractive forces holding the atoms together. The stored chemical potential energy in the bonds is released again when the water molecule is broken apart.
Energy Release
Chemical reactions involve the breaking and forming of chemical bonds. Energy is released when new bonds are formed between atoms or molecules in what are called exothermic reactions. The products of exothermic reactions have less potential energy than the reactants. This is because the new bonds formed in the products are typically lower in energy than the bonds that were broken in the reactants.
For example, when hydrogen and oxygen gases combine to form water, energy is released in the form of heat and light. The O-H bonds formed in water molecules release more energy than it takes to break the H-H and O-O bonds in hydrogen and oxygen molecules. The excess energy is given off as heat and light. Other common exothermic reactions include combustion reactions that involve the burning of fuels. In these reactions, heat and light are produced when carbon-carbon and carbon-oxygen bonds form in carbon dioxide and water from the reactant molecules.
Calculating Changes
One way to calculate the energy changes in chemical reactions is by using bond energies. Each type of chemical bond has a standard bond energy associated with it based on the strength of the bond. For example, a C-H bond has a bond energy of 413 kJ/mol, while a C-C bond has a bond energy of 347 kJ/mol.
To find the overall energy change in a reaction, you can add up the energy required to break existing bonds and subtract the energy released when new bonds are formed. This gives you the net energy change for the reaction. Exothermic reactions will have a negative net energy change, while endothermic reactions will have a positive net energy change.
For example, for the reaction:
CH4 + 2O2 → CO2 + 2H2O
You would calculate:
Bonds broken:
4 C-H bonds = 4 x 413 kJ/mol = 1652 kJ/mol
2 O=O bonds = 2 x 498 kJ/mol = 996 kJ/mol
Bonds formed:
2 C=O bonds = 2 x 799 kJ/mol = 1598 kJ/mol
4 O-H bonds = 4 x 463 kJ/mol = 1852 kJ/mol
Net energy change = (Bonds formed) – (Bonds broken)
= (1598 + 1852) – (1652 + 996)
= -802 kJ/mol
The negative net energy change indicates this is an exothermic reaction.
Reaction energy diagrams are another useful way to visualize the energy changes in a chemical reaction. These diagrams plot the total energy of the reactants and products over the course of the reaction. The activation energy, or the energy needed to start the reaction, can be seen as the peak of the diagram. The difference between the starting and final energy levels gives the enthalpy change for the overall reaction.
Applications
Chemical potential energy has many important real-world applications in various fields and technologies.
Batteries and Fuel Cells
Batteries and fuel cells convert chemical potential energy into electrical energy through electrochemical reactions. In batteries, chemical reactions occur spontaneously to generate electricity. In fuel cells, a continuous supply of fuel and oxidant drive the chemical reaction to produce electricity.
Explosives and Propellants
The large amount of potential energy stored in the chemical bonds of explosives and propellants can be rapidly released through combustion reactions. This produces a rapid expansion of hot gases that can be harnessed to do mechanical work, such as propelling a bullet or rocket.
Biological Processes
In living organisms, chemical potential energy is stored in the bonds of large molecules like carbohydrates, fats, and proteins. This energy is released through metabolic processes to power essential biological functions like muscle contraction, nerve impulses, and protein synthesis.
Kinetic Energy Conversion
Chemical energy stored in molecules can convert into kinetic energy when chemical bonds are broken. Kinetic energy is energy of motion that gets transferred to atoms, molecules or larger particles as they speed up during a chemical reaction.
For example, combustion reactions like burning fuels involve breaking chemical bonds in the fuel molecules. This releases energy that gets transferred as kinetic energy to the reaction products, causing the gases produced to accelerate rapidly and expand. The hot, expanding gases can then be used to drive mechanical processes like operating car engines.
In addition to kinetic energy, chemical reactions that break bonds also typically give off heat and light as byproducts. These are other forms of energy that are released when the chemical energy stored in substances gets converted through chemical reactions. The total quantity of chemical energy converted will be split between generating kinetic energy of motion, as well as thermal energy and electromagnetic radiation.
Thermodynamics
Chemical potential energy is closely related to Gibbs free energy in thermodynamics. The Gibbs free energy represents the maximum useful work that can be extracted from a system at constant temperature and pressure. Reactions that decrease Gibbs free energy are thermodynamically spontaneous and can occur without an input of energy.
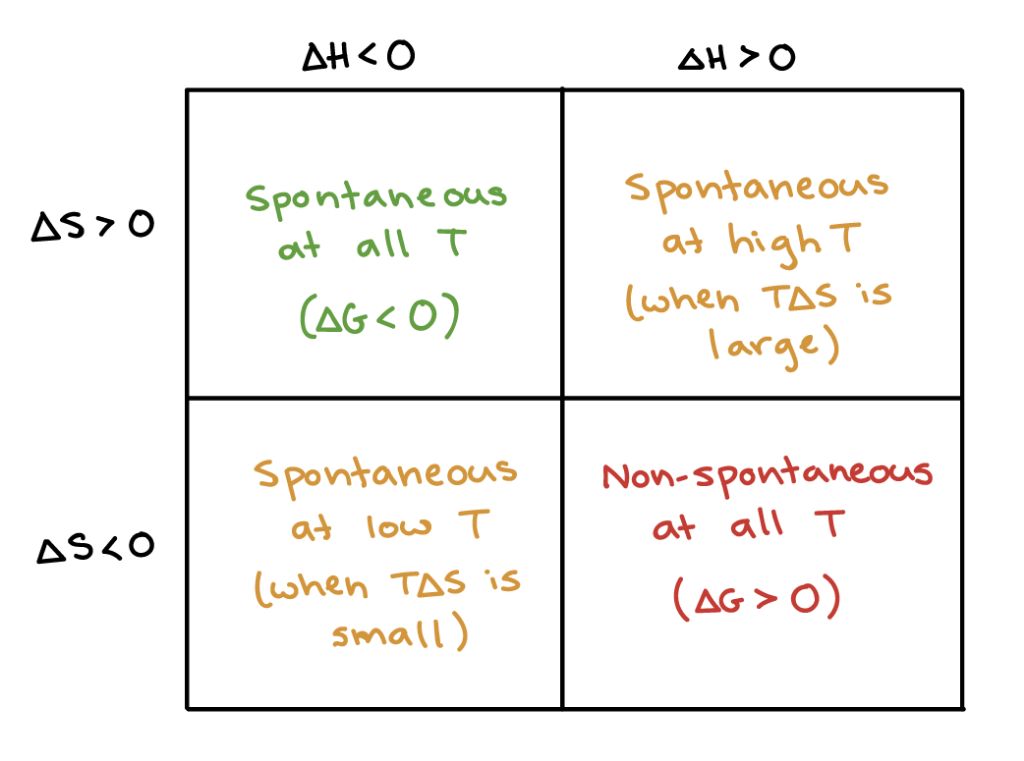
For a chemical reaction at constant temperature and pressure, the change in Gibbs free energy (ΔG) determines the potential for the reaction to occur spontaneously. Reactions with a negative ΔG will proceed spontaneously, releasing excess energy as heat. Reactions with a positive ΔG require an input of energy to overcome the energy barrier and proceed. The chemical potential energy stored in the bonds of reactants contributes to the overall change in Gibbs free energy.
Conclusion
In summary, chemical potential energy is a type of energy stored in chemical bonds. The quantity of this energy depends on the types of bonds and arrangements of atoms. This form of potential energy can be released during chemical reactions as useful kinetic energy, heat, and light.
Understanding chemical potential energy and how it converts to other forms is essential in chemistry and physics. Knowledge of chemical energy storage allows scientists to predict reaction outcomes, optimize chemical processes, and harness energy sources ranging from food to rocket fuels.