What Is Relation Between Temperature And Heat?
Definition of Temperature
In physics, temperature is a measure of the average kinetic energy of the molecules or atoms in a substance. It refers to how hot or cold an object feels. Temperature is measured using a thermometer and different temperature scales such as Celsius, Fahrenheit, and Kelvin.
The most commonly used units for temperature are:
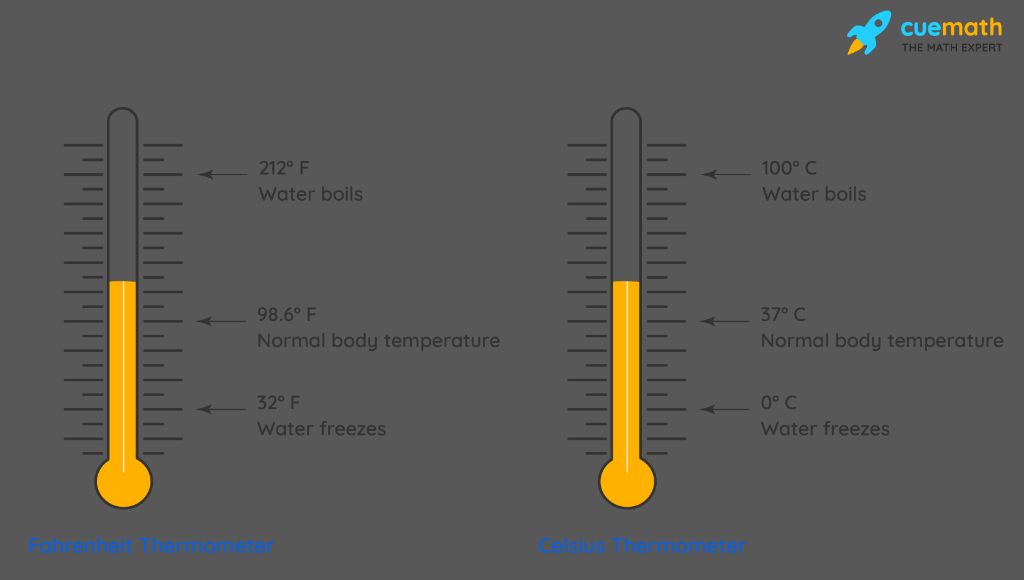
- Celsius – The Celsius scale sets 0°C at the freezing point of water and 100°C at the boiling point of water at sea level air pressure.
- Fahrenheit – The Fahrenheit scale sets 32°F as the freezing point of water and 212°F as the boiling point of water at sea level air pressure.
- Kelvin – The Kelvin scale is the standard unit of temperature used in science. On the Kelvin scale, 0 K represents absolute zero, the theoretical point where molecular motion stops. The size of 1 Kelvin is the same as 1 Celsius degree.
Definition of Heat
Heat is a form of energy that is transferred from one object to another due to a temperature difference. Heat energy causes microscopic motions and vibrations in atoms and molecules. The standard SI units of heat are joules (J) or calories (cal).
Unlike temperature, which is a property of matter that indicates the intensity of molecular motion, heat is the total thermal energy present in matter. When two objects at different temperatures come in contact, heat flows spontaneously from the hotter object to the colder one until they reach thermal equilibrium.
Heat can be defined as energy in transit solely due to temperature difference. It can be transferred between objects through conduction, convection or radiation. Heat is measured by the amount of energy transferred, while temperature measures the average kinetic energy of molecules and atoms.
Thermal Equilibrium
Thermal equilibrium refers to when two or more objects in contact reach the same temperature. Thermal equilibrium occurs because heat flows from warmer objects to cooler objects until the objects have the same temperature. When objects are in thermal equilibrium, there is no net heat transfer between them.
For example, if you have a hot cup of coffee and place it on a table, over time the coffee will lose heat to the table and air, while the table gains heat from the coffee. Eventually, the coffee, table, and surrounding air reach the same temperature – a state of thermal equilibrium. Even though the objects are still composed of moving particles with kinetic energy, there is no overall heat flow once thermal equilibrium is reached.
Thermal equilibrium is an important concept for understanding heat transfer. It explains why heat moves from hot to cold objects until they reach the same temperature. Thermal equilibrium also helps quantify heat transfer between objects based on their masses, specific heat capacities, and initial temperature differences.
Temperature and Kinetic Energy
Temperature is directly related to the average kinetic energy of molecules. Kinetic energy is the energy of motion – in this case, the motion and vibration of atoms and molecules. As temperature increases, molecules vibrate and move faster as they gain more kinetic energy. Their velocity and energy distribution changes. Higher temperature means molecules have greater average kinetic energy and motion.
This is why heating a substance increases its temperature – heating adds energy which speeds up the molecules. Cooling removes energy and slows down molecular motion, decreasing temperature. A direct relationship exists between temperature and average molecular kinetic energy. Temperature serves as a measure of molecular energy, with higher temperatures indicating faster molecular vibration and movement.
Heat Transfer
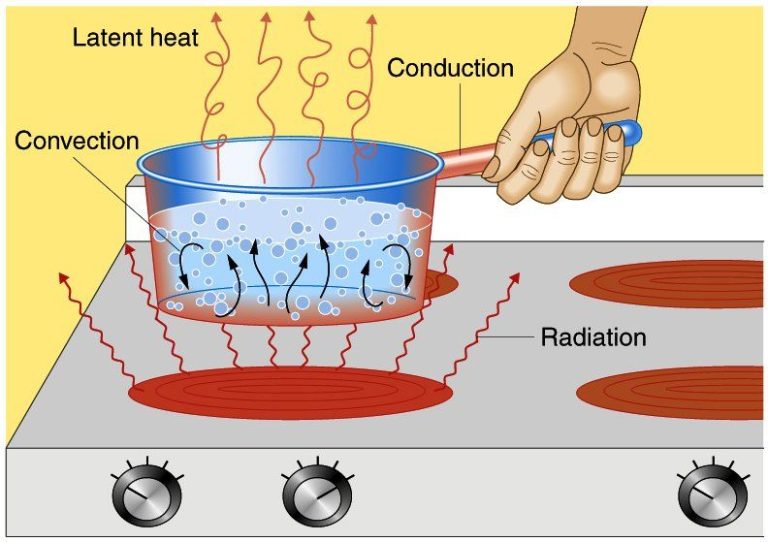
Heat transfers from one object to another in three main ways: conduction, convection, and radiation.
Conduction is the transfer of heat between objects that are in direct contact with each other. Heat is transferred between the atoms and molecules of the two objects. Metals are good conductors because their atoms can vibrate and transfer kinetic energy. Insulators have tightly bound atoms that don’t vibrate as easily, inhibiting heat transfer.
Convection is heat transfer through fluids (liquids and gases). As the fluid is heated, it expands, becomes less dense, and rises. Cooler, denser fluid then sinks to take its place, causing circulation. This movement distributes heat from hotter areas to cooler areas.
Radiation is the transfer of heat via electromagnetic waves or photons. No direct contact between objects is needed. The sun transfers warmth to the Earth through radiation. Infrared radiation from warmer objects is absorbed by cooler objects.
In general, heat flows spontaneously from objects at higher temperatures to objects at lower temperatures until thermal equilibrium is reached. The greater the temperature difference, the faster heat will be transferred.
Measuring Temperature
Temperature can be measured using various instruments called thermometers. Most thermometers measure temperature by relying on the change in some physical characteristic of a material as it gets hotter or colder. The most common types of thermometers are:
- Liquid-in-glass thermometers – These contain liquid mercury or alcohol in a glass tube. As the temperature increases, the liquid expands and rises in the tube.
- Bimetallic strip thermometers – These contain a strip made up of two metals that expand at different rates as temperature changes. This causes the strip to coil or uncoil, which is connected to a pointer to indicate the temperature.
- Thermistors – These contain materials whose resistance changes with temperature. By measuring this resistance, the temperature can be determined.
- Thermocouples – These contain two dissimilar metals joined at one end. A voltage is generated at the junction proportional to the temperature difference.
There are different temperature scales used to measure and report temperatures in various contexts:
- Celsius – Also known as centigrade. Water freezes at 0°C and boils at 100°C on this scale.
- Fahrenheit – Water freezes at 32°F and boils at 212°F on this scale. Used commonly in the United States.
- Kelvin – The SI unit of temperature with 0 K representing absolute zero. Water freezes at 273 K and boils at 373 K on this scale.
Measuring Heat
There are several ways to precisely measure heat in scientific experiments and applications:
Calorimetry is a technique used to measure the amount of heat absorbed or released during a chemical reaction or physical change. A calorimeter is a container designed to measure heat transfer. For example, an insulated container filled with a known mass of water can be used to measure the heat produced when a sample is burned inside it. The temperature change of the water multiplied by its mass and heat capacity gives the amount of heat transferred.
Heat is most often measured in units of joules in the SI system, or calories or British thermal units (BTUs) in other systems. A calorie is the amount of heat required to raise 1 gram of water by 1°C. A BTU is the amount needed to raise 1 pound of water by 1°F. There are precise conversion factors between these units.
Knowing the specific heat capacity of a substance along with its mass and temperature change allows accurate calculation of heat transfer via calorimetry. This technique has applications in chemistry, physics, engineering, physiology, and other fields.
Effects of Heat
Heat has several important effects that demonstrate the relationship between heat and temperature. Two major effects are thermal expansion and state changes.
Thermal expansion refers to the tendency of matter to expand in volume when heated. As heat is added to a substance, the molecules gain kinetic energy and vibrate more intensely. This causes the distances between molecules to increase, resulting in expansion. Most substances expand linearly when heated. This expansion can be measured by the coefficient of linear expansion. Thermal expansion explains phenomena like thermometers and thermostats.
Heat also causes state changes in matter, such as melting, boiling, evaporation, and condensation. When a substance is heated to its melting point, the molecules gain enough energy to overcome intermolecular forces and transition from a solid to a liquid state. Further heating to the boiling point provides enough energy for molecules to enter the gaseous state. Heat drives the transition between solid, liquid, and gas states. The amount of heat required depends on the substance’s latent heat capacity.
Evaporation occurs when molecules on the surface of a liquid gain enough energy to enter the gaseous state. Heat fuels this transition from liquid to gas. Condensation is the reverse process, where gas transforms into liquid by losing heat. State changes like melting, boiling, evaporation, and condensation demonstrate the intimate link between heat and changes in molecular structure and energy.
Applications
Understanding the relationship between temperature and heat has numerous practical applications in everyday life and technology:
Cooking – The transfer of heat energy to food is essential for cooking. Different cooking methods like baking, frying, etc. rely on manipulating temperature to alter food. Food safety also depends on properly heating foods to destroy pathogens.
Engines – Internal combustion engines convert heat energy into mechanical work. The temperature of engine components affects performance and efficiency.
Heating, Ventilation and Air Conditioning (HVAC) – HVAC systems use differences in air temperature to control ambient temperatures in buildings and vehicles. Thermostats measure air temperature to activate heating or cooling as needed.
Weather and Climate – Temperature and heat transfer drive weather patterns and climate systems. Sunlight warms the Earth’s surface, powering convection cycles that create wind and precipitation.
Industrial Processes – Many industrial processes involve heating or cooling materials to precise temperatures to achieve desired chemical reactions or material properties.
Energy Generation – Most power plants generate electricity by heating water to convert it to steam that spins a turbine. Nuclear, coal, natural gas and solar thermal plants all rely on heat.
Medicine – The human body must maintain a proper internal temperature for health. Doctors diagnose fever by measuring elevated body temperature.
The wide-ranging uses of temperature regulation confirm how heat and temperature are fundamental to physics, chemistry, biology and technology.
Summary
Temperature and heat, though related, are distinct physical concepts. Temperature measures the average kinetic energy of molecules and atoms, whereas heat refers to the total energy transferred between objects due to a temperature difference. While temperature is an intrinsic property of a system, heat is energy in transit that flows from hot to cold objects.
Key points:
- Temperature is measured on a scale (Celsius, Fahrenheit, Kelvin) and represents the average kinetic energy of molecules.
- Heat is the transfer of thermal energy between objects and depends on factors like mass and specific heat capacity.
- Objects at the same temperature are in thermal equilibrium as no net heat transfer occurs.
- Heat flows spontaneously from higher temperature to lower temperature objects.
- Measuring changes in temperature or heat energy helps quantify heat transfer between objects.
- Heat affects states of matter, causes thermal expansion, and is essential to thermodynamic processes.
In summary, though related, temperature and heat help describe different aspects of thermal physics – temperature characterizes the internal energy state of matter, while heat deals with energy transferred between objects due to temperature differences.