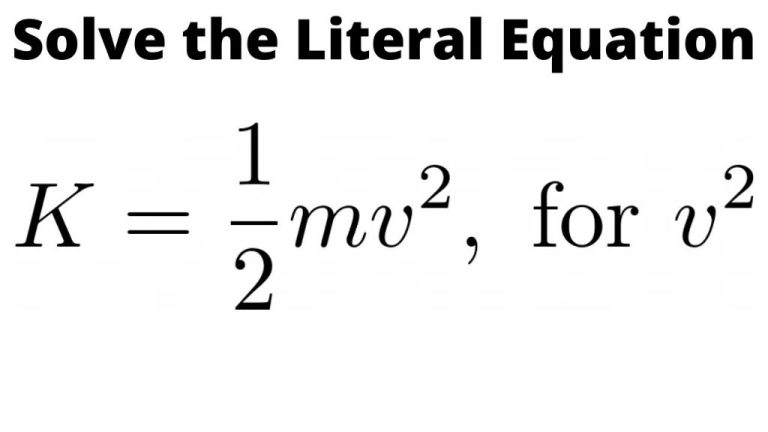What Is Meant By Potential In Chemistry?
Potential energy refers to energy stored within a system due to its configuration or position. It plays a key role in chemical reactions and thermodynamics. Chemical potential, also known as partial molar Gibbs free energy, indicates the potential of a chemical species to undergo change. Chemical potential describes how likely a reaction is to occur spontaneously and is critical for understanding chemical equilibrium.
Potential energy helps quantify interactions between molecules and predict the favorability of chemical processes. Calculating and analyzing chemical potentials provides key insights into reaction tendencies, phase changes, battery voltage, membrane transport, and more. This article will explore the meaning and applications of potential in chemistry.
Potential Energy
Potential energy is the stored energy an object has due to its position or chemical configuration. There are several types of potential energy:
- Gravitational potential energy – This is the energy stored in an object due to gravity. For example, a book held above the ground has gravitational potential energy due to the force of gravity pulling it down.
- Elastic potential energy – Energy stored in elastic materials when they are stretched or compressed. For example, a stretched rubber band has elastic potential energy.
- Electric potential energy – Energy stored in an electric field. Charged particles have electric potential energy due to their charge and proximity to other charges.
- Chemical potential energy – Energy stored in the chemical bonds between atoms and molecules. This energy can be released in chemical reactions.
In physics, an object’s potential energy represents its capacity to do work as a result of its position or state. This stored energy can be converted into kinetic energy when released.
Chemical Potential
Chemical potential refers to the potential energy of a chemical substance to undergo a change or to drive a chemical reaction. It quantifies a chemical’s tendency to change its state based on its present conditions, such as temperature, pressure, and concentration.
Chemical potential is closely related to the general concept of potential energy in physics. Potential energy describes the energy stored in a system due to its configuration or composition. Similarly, chemical potential represents the potential energy available for a chemical substance to do work.
The chemical potential of a substance determines in which direction chemical reactions will proceed. Reactions will tend to move towards states of lower chemical potential. For example, gas particles have higher chemical potential than liquid particles of the same substance. So a gas will spontaneously condense into a liquid, releasing energy in the process.
Chemical potential also indicates how systems will shift to reach equilibrium. Equilibrium is the state where the chemical potentials of all substances are equal. So a system with substances at different chemical potentials will undergo reactions and transfers until equilibrium is achieved.
Gibbs Free Energy
Gibbs free energy (G) is a thermodynamic property that is very important for understanding chemical potential and spontaneity. The Gibbs free energy of a system is defined as:
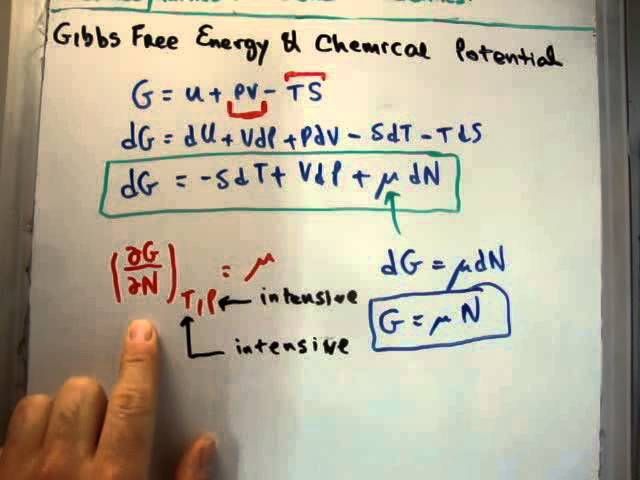
G = H – TS
Where H is the enthalpy, T is the absolute temperature, and S is the entropy. The Gibbs free energy represents the amount of energy that can be extracted as useful work from a system at constant pressure and temperature.
For any process, a negative change in Gibbs free energy (ΔG < 0) indicates that the process will occur spontaneously. This is because a negative ΔG means the system can release energy by being converted to a more disordered state. In contrast, a positive ΔG (ΔG > 0) means the process requires an input of energy and will not happen spontaneously.
Gibbs free energy is directly related to chemical potential, which provides a measure of how spontaneous a process will be. Substances tend to transition from states of higher chemical potential to lower chemical potential. The Gibbs free energy represents the maximum useful work that can be extracted from a system, and minimizing G drives chemical processes forward. Therefore, Gibbs free energy and chemical potential are extremely useful for understanding spontaneity in chemistry.
Calculating Chemical Potential
The chemical potential of a substance can be calculated using the following mathematical equation:
<μ = (∂G)/(∂n)
Where:
- μ is the chemical potential
- G is the Gibbs free energy of the system
- n is the number of moles of the substance
This equation states that the chemical potential of a substance is equal to the partial derivative of the system’s total Gibbs free energy with respect to the amount of that substance, at constant pressure and temperature. Intuitively, it represents how much the Gibbs free energy changes when an incremental amount of the substance is added to the system.
The key variables in calculating chemical potential are thus the Gibbs free energy, which depends on the conditions and nature of the system, and the amount of the substance in question. Measuring or calculating these quantities allows chemical potential to be determined through the equation.
Chemical Potential and Equilibrium
Chemical potential determines when a reaction reaches equilibrium. At equilibrium, the Gibbs free energy is at a minimum. For a reaction:
A + B ⇌ C + D
The equilibrium constant is:
K = ([C][D])/([A][B])
Where [ ] indicates concentration. At equilibrium:
ΔG = ΔG° + RTlnK = 0
R is the gas constant, T is temperature in Kelvin. ΔG° is the standard free energy change. Rearranging and taking the exponential:
K = exp(-ΔG°/RT)
This shows that K depends only on temperature and thermodynamic properties, not on concentrations. The equilibrium condition is met when ΔG = 0, i.e. when the Gibbs free energy is minimized. This occurs when the chemical potentials of reactants equals products:
μA + μB = μC + μD
At this point, forward and reverse reaction rates are equal, signifying dynamic equilibrium. The chemical potential determines and reflects when this equilibrium state is reached.
Applications
Chemical potential has important applications in several areas of chemistry and physics.
In electrochemistry, it determines the voltage of electrochemical cells. The cell potential is directly related to the change in chemical potential of reactants and products. Cells will spontaneously convert chemical energy to electrical energy if the change in chemical potential is negative.
For phase changes, chemical potential indicates the driving force for transitions between solid, liquid, and gas phases. Substances will change phase when the chemical potential in one phase becomes lower than another phase.
Chemical potential is also critical in determining solubility equilibria. When a solute dissolves, its chemical potential must decrease to a value matching the solvent. The concentration at which this occurs is the solubility limit.
Chemical potential differences drive diffusion, which is the spontaneous movement of molecules from areas of higher to lower potential. Many biological and chemical transport phenomena rely on diffusion down gradients of chemical potential.
In electrophysiology, the membrane potential in neurons and other cells is dependent on differences in chemical potential across the cell membrane. Ion transport alters the relative chemical potentials inside and outside the cell, creating voltage.
Membrane Potential
One important application of chemical potential in biology is the membrane potential in cells and neurons. The cell membrane separates the interior of the cell from the exterior environment and controls what can pass in and out of the cell. The cell membrane contains various ion channels and pumps that regulate the concentrations of ions like sodium, potassium, and chloride inside and outside the cell.
This difference in ion concentrations leads to a difference in the electrical potential across the membrane, called the membrane potential. Ions want to move down their concentration gradient from high to low concentration. But the membrane isolates the two sides, creating a separation of charge and voltage. The actions of the ion channels and pumps serve to maintain the membrane potential.
In neurons, rapid shifts in the membrane potential are used to create nerve impulses for cellular communication. The neuron has a resting membrane potential of around -70 mV. When the neuron receives stimulation, ion channels open, allowing positive ions to rush into the cell, depolarizing the membrane and creating an action potential spike. This voltage spike travels down the axon of the neuron, allowing it to transmit information.
The membrane potential is an example of electrochemical equilibrium, with both concentration gradients and electrical potentials influencing ion flow. The principles of chemical potential help explain how cells maintain the membrane potential through the regulated flow of ions across the semi-permeable membrane.
History
The concept of chemical potential originated in the 19th century with the development of thermodynamics. Several key scientists contributed to establishing the idea of chemical potential energy:
In 1876, the American physicist Josiah Willard Gibbs published a landmark paper called “On the Equilibrium of Heterogeneous Substances” that introduced the concept of chemical potential, or what he called “available energy.” Gibbs’ work built on previous thermodynamics research and formulated the idea that the energy available for chemical work in a system depends on the amounts and properties of substances present.
Around the same time, the German physicist Hermann von Helmholtz was developing similar ideas regarding the energy of chemical reactions and systems. In 1882, he coined the term “free energy” to describe the useful work obtainable from a chemical system, which aligned with Gibbs’ concept of available energy.
In the early 20th century, German physical chemist Walther Nernst helped integrate the concept of chemical potential into his heat theorem. Nernst showed that chemical potential was linked to system temperature and helped demonstrate how chemical potential related to equilibrium based on his studies of electrochemical systems.
Over time, the term “chemical potential” became the preferred terminology, encapsulating the energetic capacity available to induce chemical changes. The advances of Gibbs, Helmholtz, Nernst and others established chemical potential as a fundamental thermodynamic property central to analyzing chemical systems.
Conclusion
In summary, chemical potential is a very important thermodynamic concept in chemistry and physics. It represents the partial derivative of a system’s Gibbs free energy with respect to the number of particles of a component. Chemical potential provides a way to determine the direction of spontaneous processes and quantify a system’s tendency to change.
Some key points about chemical potential:
- It indicates the change in Gibbs free energy when the amount of a component is increased.
- Systems flow spontaneously from higher chemical potential to lower chemical potential.
- Equilibrium is reached when chemical potentials are equal across system boundaries.
- It allows prediction of direction of chemical reactions and phase transformations.
- Membrane and electrical potentials in biology involve gradients in chemical potential.
Overall, the concept of chemical potential is foundational in thermodynamics and is critical for understanding and predicting a wide range of chemical and physical phenomena. Appreciating chemical potential provides deep insight into the driving forces behind processes ranging from chemical reactions to the behavior of batteries and fuel cells. It is a powerful tool for elucidating nature’s tendency toward equilibrium.