What Is Kinetic Energy In Terms Of Chemistry?
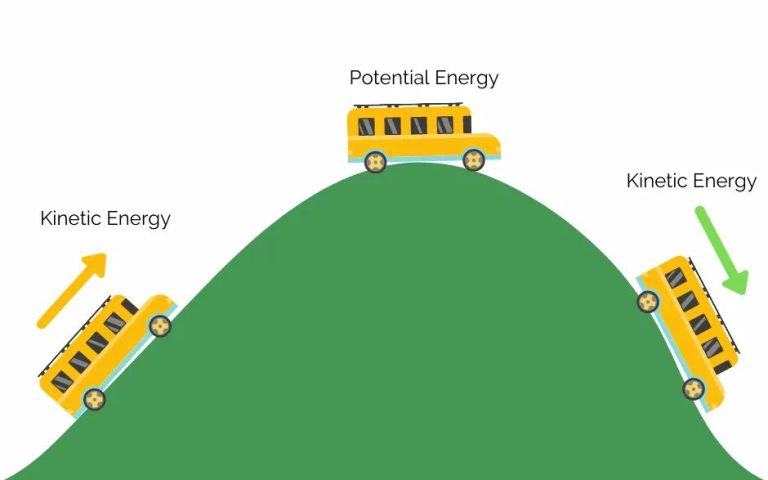
Kinetic energy is the energy associated with movement. It is directly proportional to the mass of an object and the square of its velocity. In chemistry, kinetic energy refers to the energy possessed by atoms and molecules due to their motion. The kinetic energy of atoms and molecules is a fundamental concept in chemistry with wide-ranging implications.
Kinetic energy determines the forces between molecules and their chemical reactivity. The kinetic energy of reactant molecules must be sufficient to overcome the activation energy barrier for a reaction to occur. Kinetic energy is related to temperature; higher temperatures correlate to greater molecular motion and kinetic energy. An understanding of kinetic energy is key for describing chemical kinetics, thermodynamics, molecular collisions, and the behavior of gases.
The kinetic molecular theory, based on kinetic energy concepts, explains the physical properties of gases and forms the basis for gas laws. Overall, kinetic energy provides a crucial bridge between the microscopic properties of individual atoms and molecules and the macroscopic properties and processes observed in day-to-day chemistry. Grasping kinetic energy is fundamental for explaining observations, predicting chemical phenomena, and understanding the dynamics of chemical systems.
Kinetic Energy Formula
The formula for kinetic energy is:
Ek = 1/2 mv2
Where:
- Ek = kinetic energy (in joules, J)
- m = mass (in kilograms, kg)
- v = velocity (in meters per second, m/s)
This is known as the classic formula for kinetic energy. It shows that kinetic energy is directly proportional to the mass of the object and to the square of its velocity. In other words, the faster an object moves, the more kinetic energy it possesses.
Kinetic Energy Units
Kinetic energy is commonly measured in the following units:
- Joules (J) – This is the standard SI unit for measuring energy, including kinetic energy. 1 joule is equal to the kinetic energy of an object with a mass of 1 kg moving at 1 m/s.
- Electron volts (eV) – This is a common unit in chemistry and physics, defined as the amount of energy gained by a single electron moving across an electric potential difference of 1 volt. 1 eV is equivalent to 1.602 × 10-19 joules.
- Calories (cal) – This is an older non-SI energy unit still used in some contexts. 1 calorie is the amount of energy needed to raise 1 g of water by 1°C. 1 calorie is equivalent to 4.184 joules.
- Kilocalories (kcal) – Also known as Calorie with a capital C, this is equal to 1000 calories. Food calories are actually kilocalories.
- BTUs – British thermal units are common units for measuring energy content. 1 BTU is the amount of energy needed to heat 1 pound of water by 1°F. 1 BTU is equal to about 1055 joules.
Kinetic energy values can be easily converted between these units using the appropriate conversion factors.
Kinetic Energy and Temperature
There is a direct relationship between the temperature of matter and the average kinetic energy of its particles. Temperature is a quantitative measure of the average kinetic energy of the molecules or atoms that make up a substance. As the temperature increases, the average kinetic energy of the particles increases. As the temperature decreases, the average kinetic energy decreases.
This relationship can be explained by the kinetic molecular theory, which states that matter is composed of tiny particles that are in constant motion. The temperature of a substance is proportional to the average kinetic energy of its particles. Particles with greater kinetic energy move faster. As a substance is heated, its particles move faster as they gain kinetic energy. Therefore, heating increases the temperature by increasing the average kinetic energy of the particles.
The exact relationship between temperature and average kinetic energy is given by:
KEavg = (3/2) kT
Where KEavg is the average kinetic energy of the particles, k is the Boltzmann constant, and T is the absolute temperature in Kelvin. This equation shows that as temperature (T) increases, the average kinetic energy (KEavg) also increases proportionally. The Boltzmann constant k relates the kinetic energy to the temperature in a precise way.
Understanding the link between temperature and kinetic energy is fundamental to chemistry. Many chemical processes and reactions occur faster at higher temperatures because the reacting particles have more kinetic energy to overcome activation barriers. The kinetic molecular theory and the relationship between temperature and kinetic energy provide the foundation for this effect.
Kinetic Molecular Theory
The kinetic molecular theory relates directly to kinetic energy by describing the motion and energy of molecules at the microscopic level. The theory makes the following key assumptions:
- Matter is composed of tiny particles called molecules that are in constant motion.
- The molecules are in constant random motion and collide with each other and container walls.
- The kinetic energy of the molecules is proportional to the absolute temperature of the substance.
- The molecules exert no attractive or repulsive forces on each other.
Therefore, according to the kinetic molecular theory, the kinetic energy of molecules increases with increasing temperature. At higher temperatures, molecules move faster and collide more frequently, leading to higher kinetic energy. This microscopic molecular motion and kinetic energy helps explain macroscopic properties of gases, liquids, and solids.
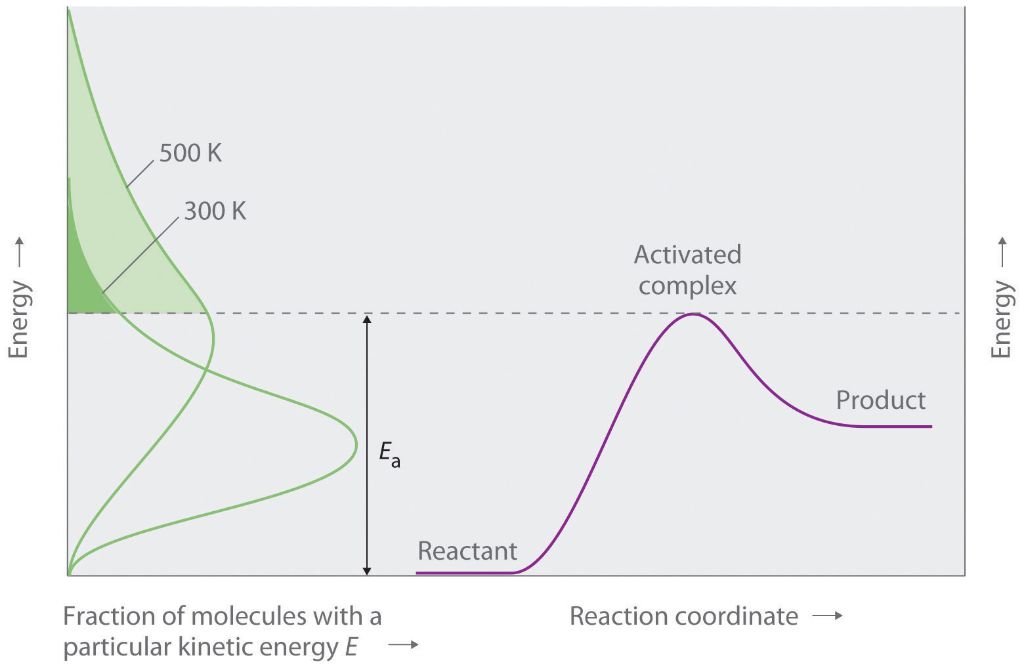
Maxwell-Boltzmann Distribution
The Maxwell-Boltzmann distribution describes the distribution of kinetic energies among molecules in thermal equilibrium at a given temperature. It relates the probability of finding a molecule with a certain kinetic energy to the temperature of the system.
As temperature increases, molecules move faster and have higher kinetic energies on average. However, at any given temperature, there is a spread of kinetic energies around the average value. The Maxwell-Boltzmann distribution mathematically describes this spread.
At low kinetic energies, the probability density function increases exponentially. As it reaches the average kinetic energy, the distribution peaks. At higher energies, the probability density drops off rapidly, according to a Gaussian function.
The most likely kinetic energy occurs at the peak of the Maxwell-Boltzmann distribution. However, higher energy tails are possible, just increasingly less probable. The distribution reflects the random, constant motion of molecules in a gas.
Understanding this distribution is key for relating temperature to molecular kinetic energies. The average kinetic energy from the Maxwell-Boltzmann distribution also allows calculating important thermodynamic variables like pressure.
Activation Energy
Activation energy is the minimum amount of energy required to start a chemical reaction. Reactions involve the breaking and formation of chemical bonds, which requires energy. Activation energy provides the necessary energy to break bonds in the reactant molecules and allows new bonds to form to create the product molecules.
For a reaction to occur, the reactant molecules must collide with sufficient energy to overcome the activation energy barrier. The activation energy is like the height of a hill that reactant molecules must climb before they can transform into product molecules and proceed downhill energetically. Molecules that do not collide with adequate energy will simply bounce off each other unchanged.
The activation energy depends on the type of chemical bonds being broken and formed during the reaction. Stronger bonds require more activation energy to break. Weaker bonds have lower activation energy barriers. The activation energy also depends on orientation – some molecular orientations during collisions allow for easier bond breaking.
In summary, activation energy is a critical concept in chemical kinetics. It explains why most chemical reactions require an input of heat or light to proceed at appreciable rates. By providing enough activation energy to start the reaction, the minimum energy barrier can be overcome and the reaction activated.
Kinetic Energy and Reaction Rates
Kinetic energy plays a key role in determining the rates of chemical reactions. The rate of a chemical reaction is a measure of how quickly reactants are converted into products. According to collision theory, molecules must collide with sufficient energy and proper orientation for a reaction to occur. The minimum amount of energy required for a reaction to proceed is called the activation energy.
Molecules that collide with energy greater than the activation energy can overcome the energy barrier and react. The fraction of molecules that have enough kinetic energy to react is determined by the Maxwell-Boltzmann distribution. At higher temperatures, molecules have more kinetic energy on average, so a larger fraction of collisions will exceed the activation energy. Thus, increasing the temperature of the reactants speeds up the reaction rate.
The Arrhenius equation gives the quantitative relationship between temperature and reaction rate. The rate constant k increases exponentially with increasing temperature. The activation energy Ea represents the temperature dependence of the reaction rate. Reactions with larger Ea values have rates that are more sensitive to temperature changes. In summary, kinetic energy determines whethermolecule collisionscan overcome the activation barrier and how rapidly reactants are converted into products.
Examples and Applications
There are many examples demonstrating the importance of kinetic energy in chemistry:
Diffusion: The movement of molecules from high to low concentration is explained by kinetic molecular theory. Molecules with higher kinetic energies can overcome intermolecular forces and move between spaces, allowing diffusion to occur.
Phase Changes: Kinetic energy determines whether a substance is a solid, liquid or gas. Solids have lower average kinetic energy, liquids have more energy and can flow, while gases have very high kinetic energy and freely move around.
Reaction Rates: Reactions require collisions between reactant molecules. Molecules with higher kinetic energy collide more often and more forcefully, increasing the reaction rate.
Catalysts: Catalysts work by reducing the activation energy barrier, allowing more reactant molecules to have enough kinetic energy to undergo successful collisions and reactions.
Temperature Effects: Heating increases molecular kinetic energy, causing faster diffusion, more evaporation, and higher reaction rates. Cooling has the opposite effect.
Gas Laws: The relationship between temperature, pressure, volume and kinetic energy underlies gas behavior described by gas laws like Boyle’s Law and Charles’ Law.
Summary
Kinetic energy is a key concept in chemistry, especially in understanding chemical reactions. The kinetic energy of molecules determines how likely they are to react and how quickly reactions will proceed.
Some key points about kinetic energy in chemistry include:
- Kinetic energy refers to the energy of motion of molecules. It depends on the mass and velocity of molecules.
- The kinetic energy distribution of molecules in a sample determines its temperature. Higher temperature means higher average kinetic energy.
- The kinetic molecular theory describes the behavior of gases based on the kinetic energy of gas molecules.
- The Maxwell-Boltzmann distribution gives the probability distribution of molecular kinetic energies at a given temperature.
- Activation energy is the minimum kinetic energy molecules need to undergo a chemical reaction.
- Increasing temperature increases average kinetic energy, allowing more molecules to exceed the activation energy and react.
- Understanding how kinetic energy affects reaction rates has many applications in chemistry and chemical engineering.
By grasping the principles of molecular kinetic energy, we gain powerful insights into chemical reactivity and dynamics.