What Is Energy Made Of?
Defining Energy
Energy is the ability to do work or cause change. It exists in many forms. According to the physics stack exchange, energy is a conserved quantity associated with a time translation symmetry in the Lagrangian of a system.
As explained on Science Forums, energy is a property of a physical system where the system may involve matter and/or fields. Energy allows work to be done or transformations to occur.
The medical dictionary defines energy in physics as the capacity to do work or produce heat. It exists in potential, kinetic, thermal, electrical, and other forms (Medical Dictionary).
Forms of Energy
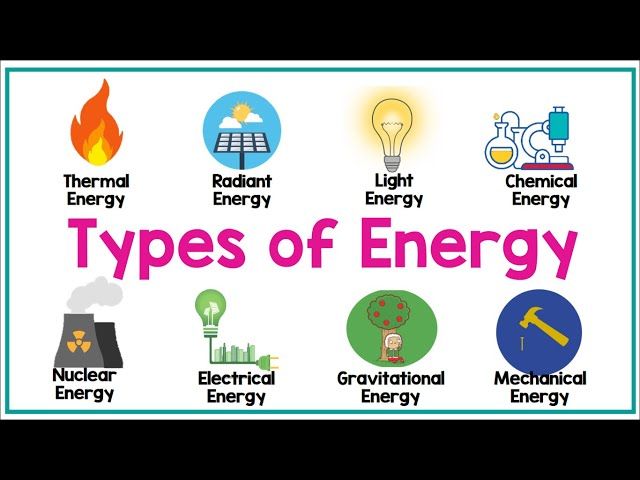
Energy comes in many different forms. Some of the main forms of energy include:
Potential Energy
Potential energy is stored energy due to an object’s position or arrangement. For example, a ball held at a height above the ground has gravitational potential energy due to gravity’s influence on the ball (2.3b Forces and Types of Energy). Other examples include energy stored in springs and chemical bonds.
Kinetic Energy
Kinetic energy is the energy of motion. A moving object has kinetic energy determined by its mass and velocity. For instance, a soccer ball flying through the air has kinetic energy that can be transferred upon impact.
Chemical Energy
Chemical energy is energy stored in the bonds between atoms and molecules. Chemical reactions release this energy when bonds are broken and rearranged. Examples include the energy stored in batteries, food, fuel, and explosives.
Thermal Energy
Thermal energy, or heat, is the total kinetic energy of molecules within an object. Adding heat increases molecular motion, while removing heat decreases this internal motion. All objects contain some thermal energy related to their temperature.
Electrical Energy
Electrical energy results from the flow of electrons. This energy powers electric currents and is used in appliances, computers, lights, and other electronics. Batteries and generators convert other forms of energy into electrical energy.
Nuclear Energy
Nuclear energy comes from processes that convert matter into energy, including nuclear fission, fusion, and radioactive decay. This energy is released from the nuclei of atoms and can be harnessed for electricity generation.
Matter and Energy
Matter is anything that has mass and takes up space. According to the Energy and Matter guide, energy is a property of matter. Objects that have matter also have energy. Matter and energy are intrinsically linked. As explained on Quora, the relationship between matter and energy is described by Einstein’s famous equation E=mc^2. Here, E represents energy, m represents mass, and c is the speed of light. This equation shows that energy and matter are interchangeable.
Matter can be converted into energy, and energy into matter. For example, the chemical energy stored in food is converted to thermal and kinetic energy in the body. During nuclear reactions, a small amount of matter is converted into a massive amount of energy. According to Quizlet flashcards, any substance that has mass and occupies space is matter. Energy is the ability to do work or cause change. Without matter, there would be no energy.
Energy Transformations
Energy can be converted or transformed from one form into another, but it cannot be created or destroyed. This principle is known as the First Law of Thermodynamics. For example, a fireplace transforms the chemical energy in wood into thermal energy and light energy. The laws of thermodynamics describe how energy is transferred and transformed.
Some common examples of energy transformations include:https://www.softschools.com/examples/science/energy_transformations_examples/161/
- A toaster transforms electrical energy into thermal energy by heating up bread
- A light bulb transforms electrical energy into light energy and thermal energy
- A bicycle transforms chemical energy from food into mechanical energy of motion
- Solar panels transform radiant light energy from the sun into electrical energy
Energy transformations happen continuously all around us, from the chemical reactions within our cells to the lighting of a match. The variety of energy forms provides an abundance of ways to utilize energy for human purposes.
Potential Energy
Potential energy is the stored energy in an object due to its position or configuration. For example, the potential energy of water held behind a dam is the energy that will be released once the water is allowed to flow. Other common examples include objects held above the ground, compressed springs, charged batteries, and separated electric charges. According to classical physics, potential energy is defined as the negative of the work done by a conservative force moving an object from an initial reference position to its current position.
The formula for potential energy depends on the situation, but some common ones include:
- Gravitational potential energy = mgh, where m is mass, g is acceleration due to gravity, and h is height.
- Elastic potential energy = 1/2kx^2, where k is the spring constant and x is the displacement from equilibrium.
- Electric potential energy = qV, where q is the charge and V is the voltage.
Potential energy is converted into kinetic energy when the object is allowed to move, such as a rock falling or a compressed spring expanding. Understanding potential energy has many practical applications, from generating hydroelectric power to designing structures stable enough to withstand environmental forces.
Sources:
https://physics.stackexchange.com/questions/696605/potential-energy-definition
https://school.careers360.com/physics/potential-energy-topic-pge
Kinetic Energy
Kinetic energy is the energy an object has due to its motion. It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes. The same amount of work is done by the body when decelerating from its current speed to a state of rest.
In classical mechanics, the kinetic energy of a non-rotating object of mass m traveling at a speed v is \frac{1}{2} mv^2. In relativistic mechanics, this is only a good approximation when v is much less than the speed of light.
Kinetic energy is an expression of the fact that a moving object can do work on anything it hits; it quantifies the amount of work the object could do as a result of its motion. This enables predictions of the severity of an impact from the kinetic energy of a moving object before the impact occurs. In gravitational physics, the energy needed to lift an object a given height, and the energy released when the object falls, are both forms of potential energy associated with the gravitational field, rather than expressions of the kinetic energy changes during the process.
For more information see:
Kinetic Energy Physics | Rakesh Pandey – YouTube
Chemical Energy:
Chemical energy is energy stored in the bonds between atoms and molecules. It is the energy released when a chemical compound reacts or changes shape. For example, the food we eat contains chemical energy that is released when we digest it. This provides energy for our cells to function and our bodies to move. The gasoline in a car and the battery in a phone also store chemical energy that is released to power mechanical processes.
Chemical energy is converted into other forms of energy during chemical reactions. When a chemical compound undergoes a chemical reaction, old bonds are broken and new bonds are formed, resulting in new chemical compounds. Breaking chemical bonds requires energy, while forming bonds releases energy. The amount of energy released or consumed depends on the strength of the chemical bonds. Strong chemical bonds store more chemical energy than weak bonds.
For example, the combustion of gasoline is a chemical reaction that breaks bonds in the gasoline molecules and forms new bonds with oxygen, producing carbon dioxide and water. This chemical reaction releases heat energy that can be used to power a car engine. The amount of available chemical energy in the gasoline limits the amount of mechanical work the car can do before needing more fuel. Other examples of chemical energy being converted to other forms include metabolism in cells, batteries, and explosives.
2024 Anti allergy medication for dogs
Thermal Energy
Thermal energy refers to the internal energy associated with the random motion of particles (molecules and atoms) in matter. All matter consists of particles that are in constant motion. The faster the particles move, the higher the thermal energy. Thermal energy is directly proportional to the temperature of matter – the greater the temperature, the greater the thermal energy. This internal energy is referred to as heat energy or simply heat.
While thermal energy refers to the total energy of particle motion within a substance, heat refers specifically to the transfer of that thermal energy between substances or within a single substance. Heat flows spontaneously from a region of higher temperature (higher thermal energy) to a region of lower temperature (lower thermal energy). This transfer of thermal energy occurs through conduction, convection or radiation until thermal equilibrium is reached.
So in summary – thermal energy is the internal kinetic energy associated with particle motion, while heat refers to the transfer of that energy. Thermal energy is contained within a system whereas heat is energy in transit. An increase in thermal energy manifests itself as an increase in temperature of the system.
Sources:
https://garyturnerscience.com/Yr%209%20Science/Term%203%20Energy,%20Electricity,%20and%20Light/1.%20Heat%20energy/ANSWERS%20Heat%20transfer%20booklet.docx
Electrical Energy
Electrical energy refers to the energy derived from the movement of electric charges or electrons. It arises from the electric potential energy of charged particles caused by the electric field, or from the kinetic energy of moving charges. When charges move through a potential difference, they lose potential energy and gain kinetic energy. This kinetic energy is converted into other forms like heat, light, or mechanical work.
Electrical energy is extremely versatile and widely used in modern society. It powers all electric appliances, lights, computers, and industrial equipment. Electrical energy can be easily converted into other useful forms of energy via electromechanical devices like motors, generators, and transformers. For example, an electrical heater converts electrical energy into heat, while a light bulb changes it into light energy. Electricity allows energy to be transmitted over long distances, enabling widespread distribution of energy through power grids.
Common sources of electrical energy include batteries, which convert chemical energy into electricity through redox reactions, and generators like hydroelectric dams or wind turbines, which use mechanical energy to move a magnet through a coil of wire and generate electricity via electromagnetic induction. Controlling and utilizing electrical energy via circuits, electronics and electromagnetism has enabled the development of essential technologies like telecommunications, computing, transportation, and more in the modern world.
According to LibreTexts, electrical energy represents “the ability to do work by moving electrons through an external potential difference”. Understanding how to generate, harness and convert it is key for utilizing electricity in useful ways.
Nuclear Energy
Nuclear energy is the energy stored in the nucleus of an atom. It is released when the nucleus undergoes nuclear reactions such as nuclear fission or fusion. In nuclear fission, the nucleus of an atom like uranium or plutonium splits into smaller nuclei, releasing a large amount of energy. Nuclear power plants use nuclear fission to generate electricity. In nuclear fusion, two or more atomic nuclei fuse together to form a heavier nucleus, releasing energy in the process. The sun produces energy through nuclear fusion of hydrogen nuclei.
Nuclear energy has an extremely high energy density, meaning a small amount of fuel can produce vast amounts of energy. Uranium, for example, has over a million times more energy per unit mass than fossil fuels like coal and oil. However, nuclear power also produces radioactive waste that must be carefully contained and managed. There are risks associated with nuclear accidents and the proliferation of nuclear weapons. Proponents argue nuclear power emits zero greenhouse gases during operation and is a low-carbon energy source that can help address climate change. Critics counter that lifecycle emissions including mining, enrichment, and plant construction are significant.
Overall, nuclear power accounts for around 10% of the world’s electricity generation. As of 2021, there are over 400 nuclear power reactors operating in over 30 countries around the world. Nuclear energy remains controversial but provides a sizeable source of reliable, low-carbon baseload electricity in many regions.